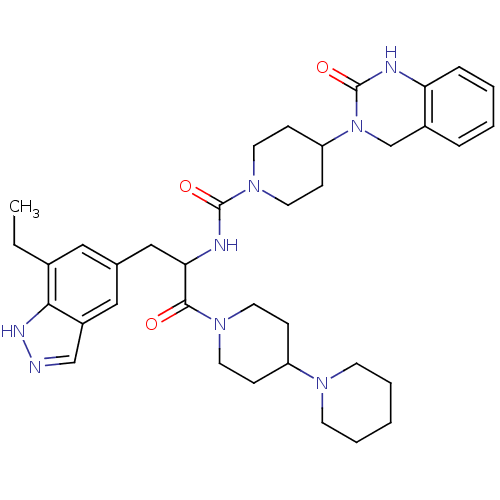
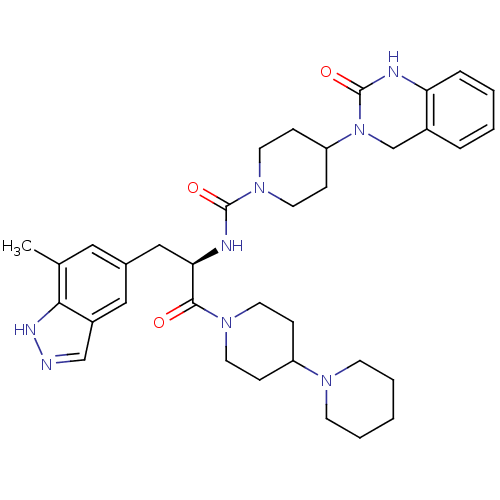
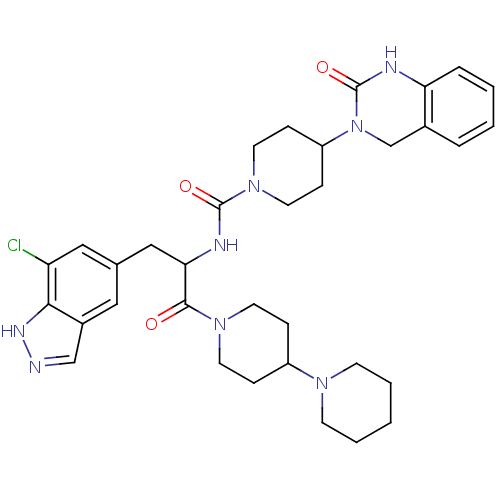
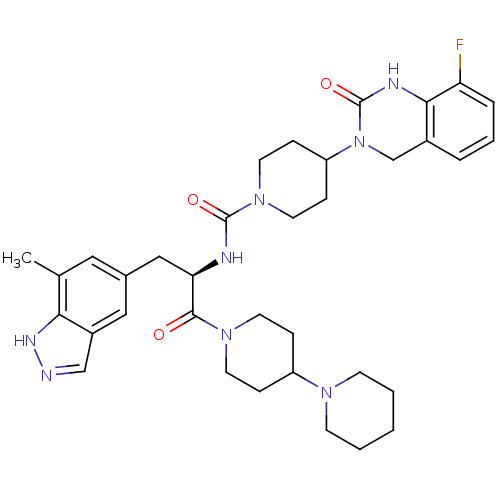
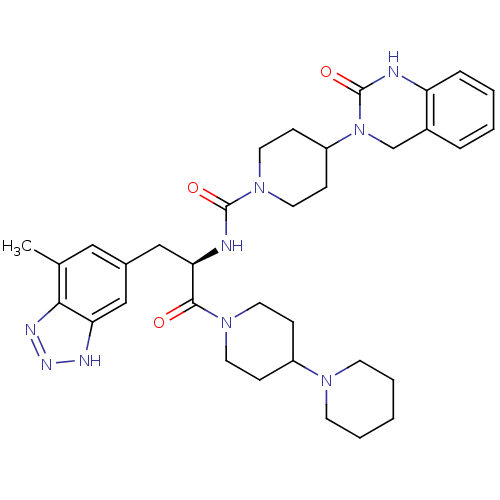
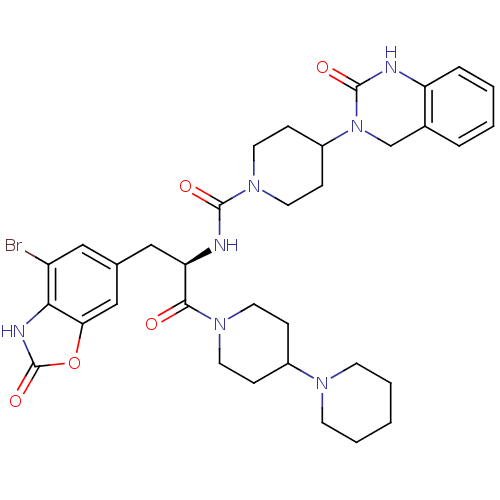
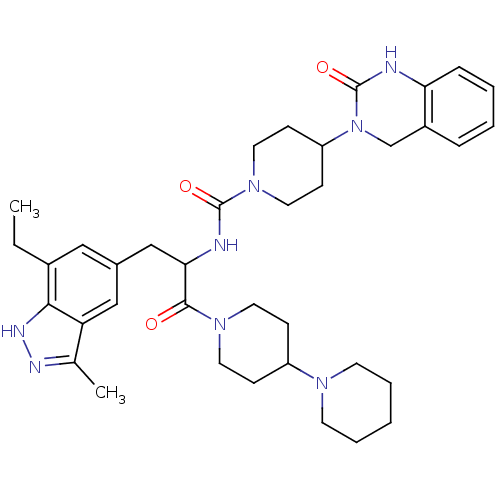
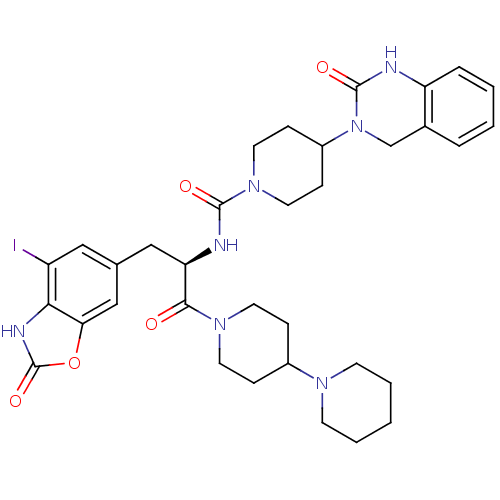
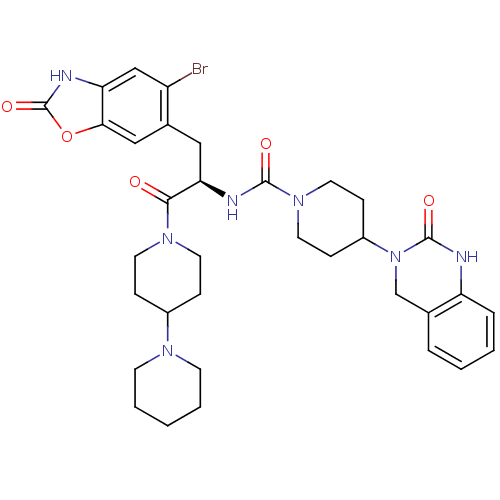
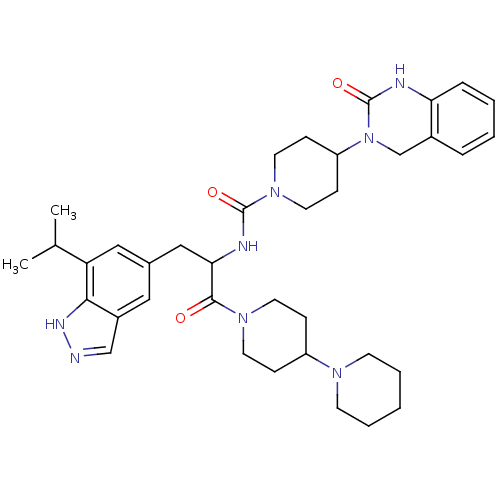
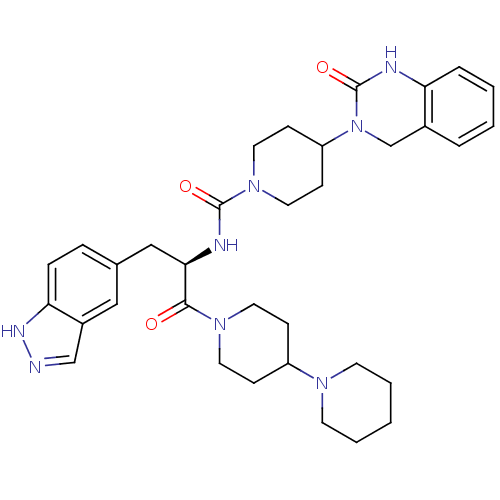
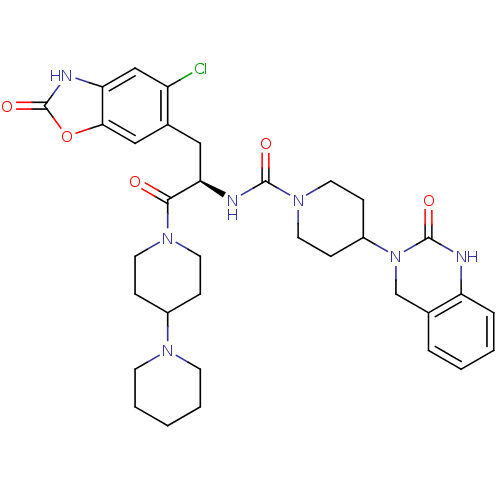
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
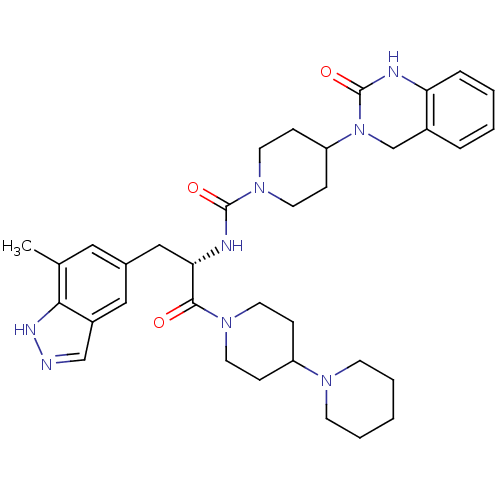
Affinity DataKi: 0.00500nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 0.00730nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0110nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0120nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0130nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0150nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0230nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0320nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0390nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0480nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0550nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 0.140nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 0.220nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 0.230nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 0.280nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCalcitonin gene-related peptide type 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataKi: 27nMAssay Description:Binding affinity to CGRP receptor (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-resorufin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 370nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-resorufin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 450nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-resorufin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-resorufin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-resorufin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-resorufin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-4-trifluoromethylcoumarin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-resorufin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-resorufin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-resorufin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-resorufin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 6.70E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-resorufin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 6.90E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-resorufin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 7.20E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-resorufin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 7.30E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-resorufin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-4-trifluoromethylcoumarin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-resorufin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-4-trifluoromethylcoumarin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.40E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-4-trifluoromethylcoumarin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-4-trifluoromethylcoumarin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.60E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-4-trifluoromethylcoumarin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.20E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-4-trifluoromethylcoumarin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.60E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-4-trifluoromethylcoumarin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-4-trifluoromethylcoumarin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-4-trifluoromethylcoumarin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-4-trifluoromethylcoumarin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-4-trifluoromethylcoumarin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-4-trifluoromethylcoumarin as substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using 7-benzyloxy-4-trifluoromethylcoumarin as substrateMore data for this Ligand-Target Pair