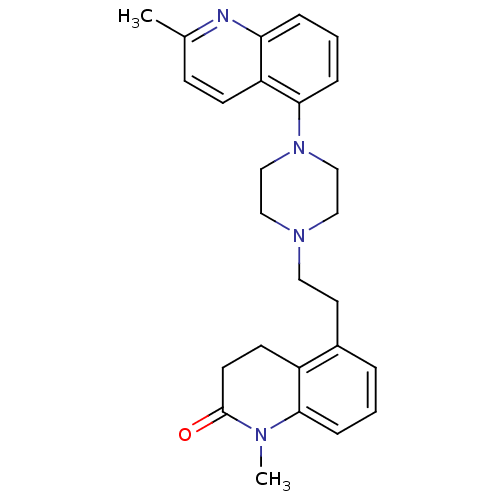
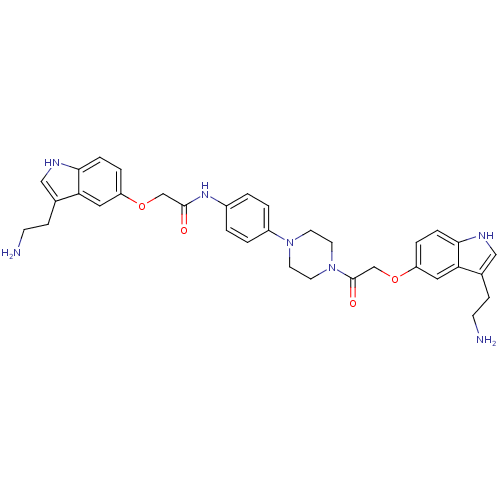
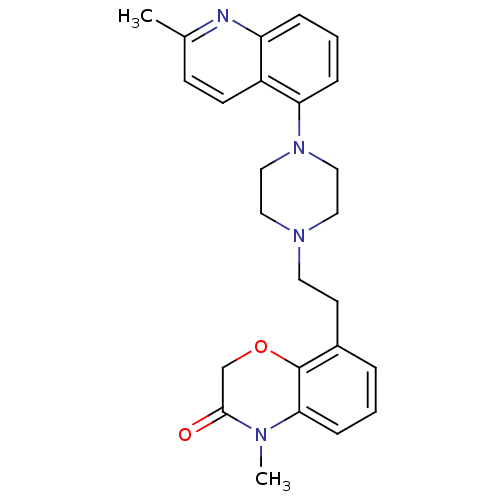
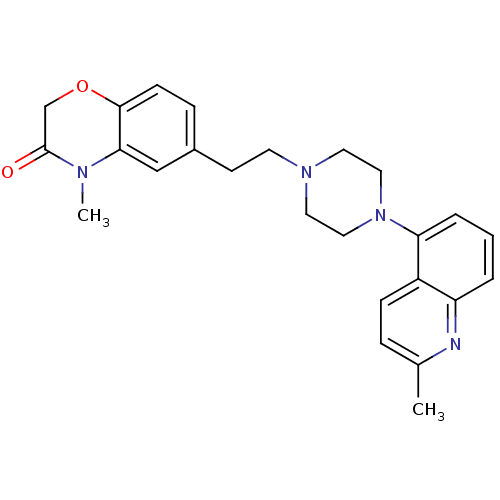
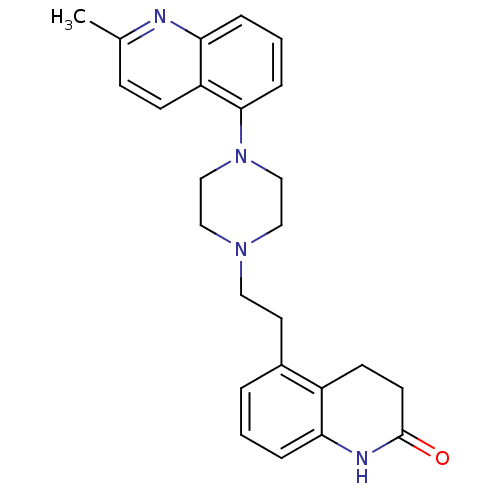
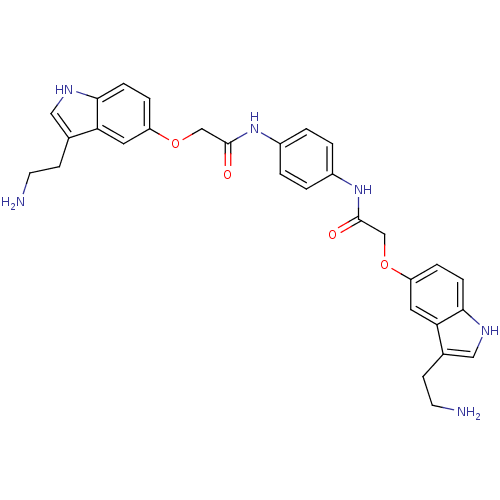
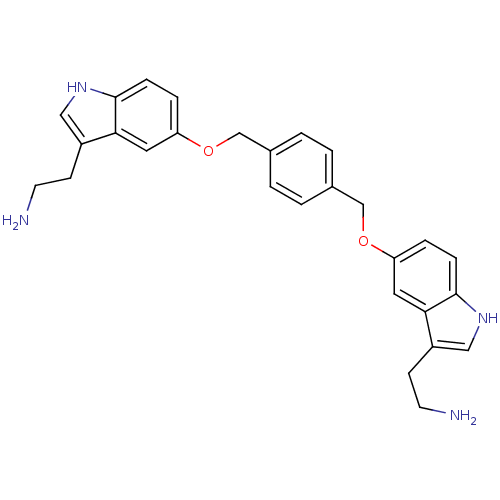
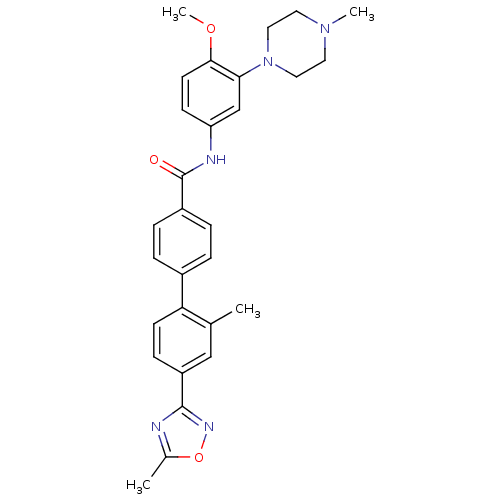
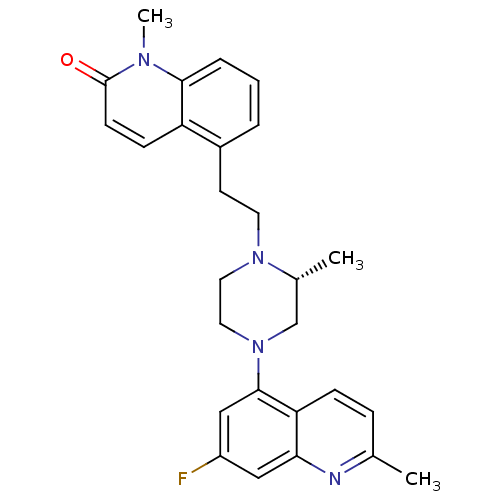
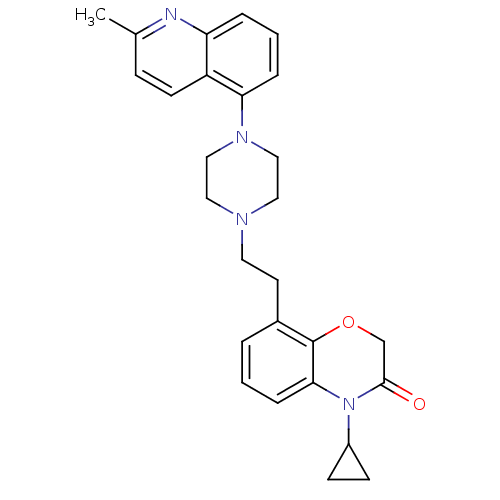
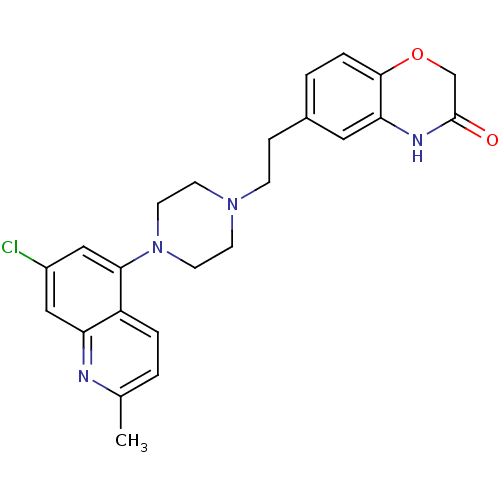
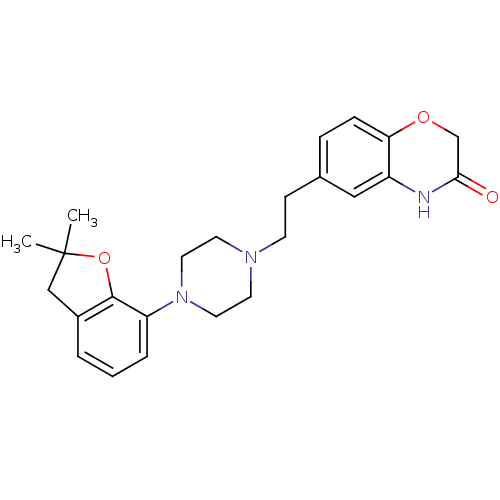
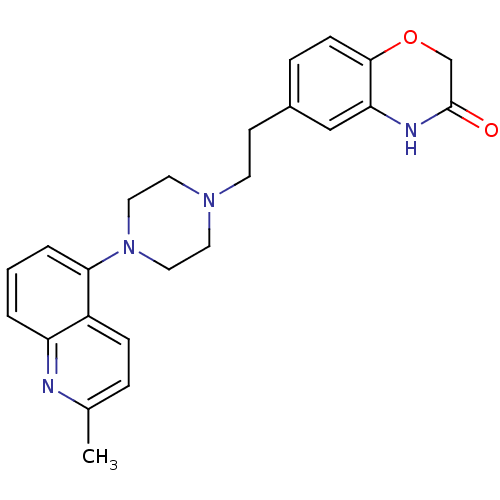
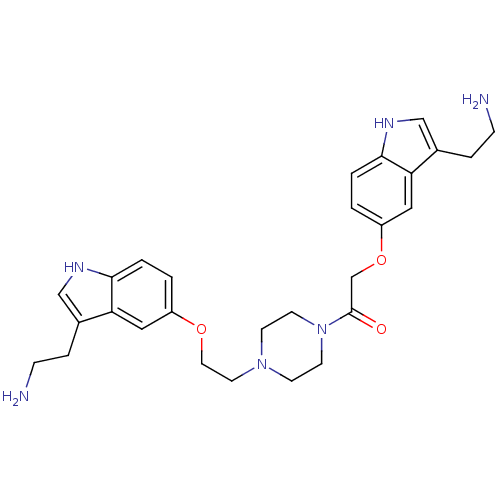
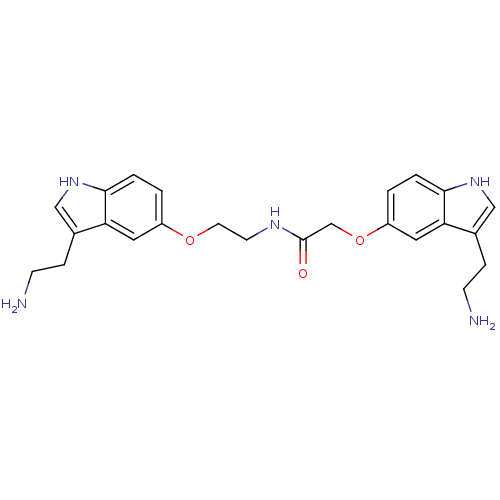
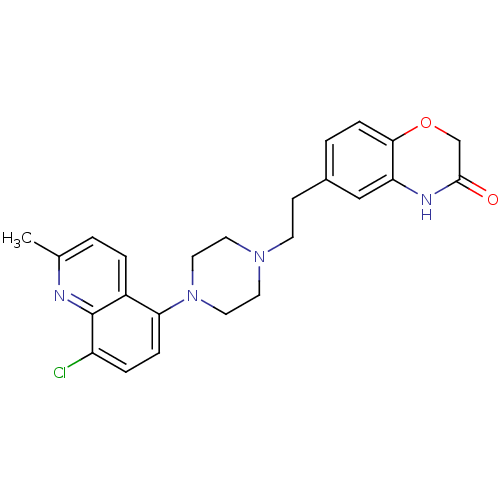
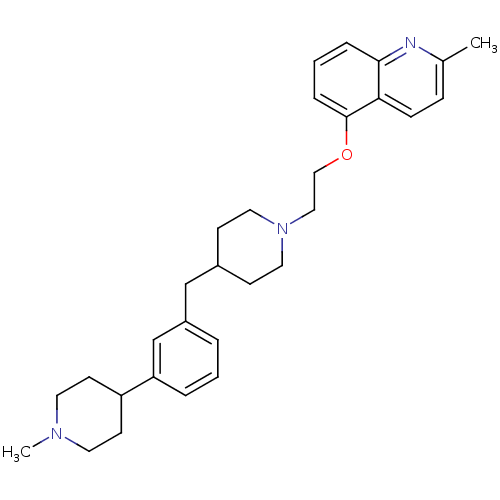
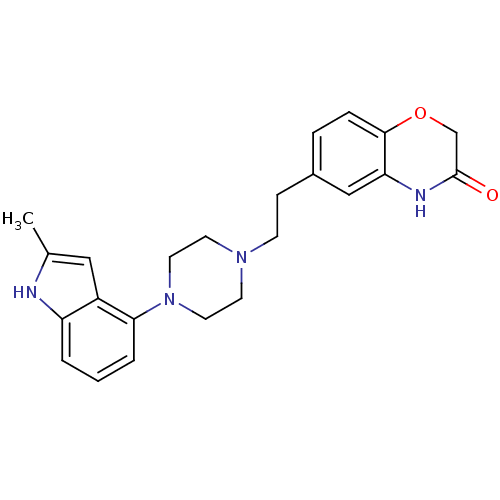
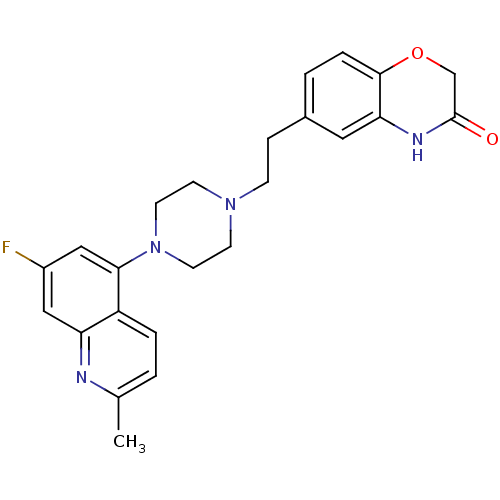
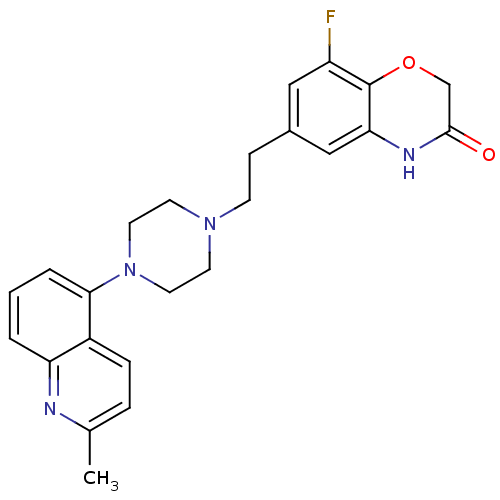
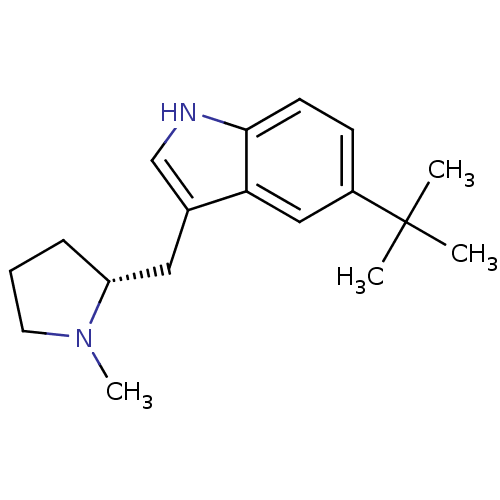
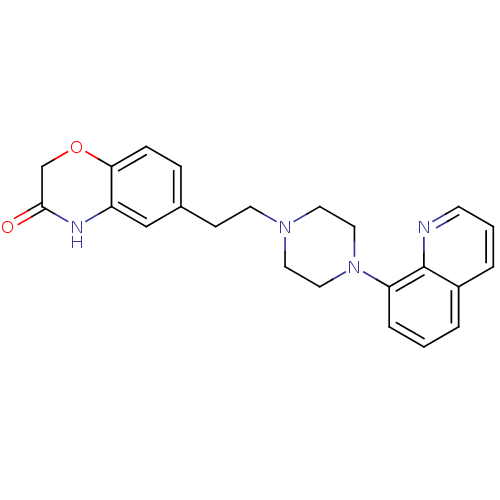
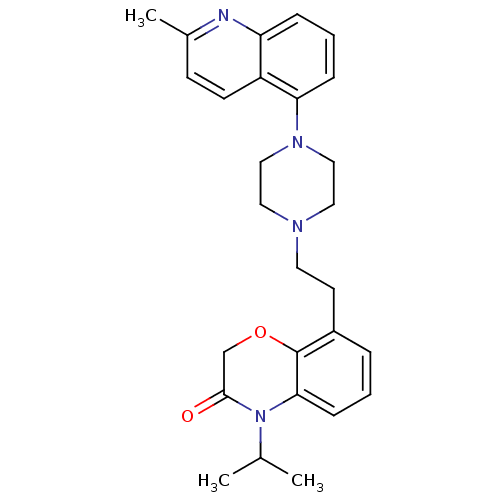
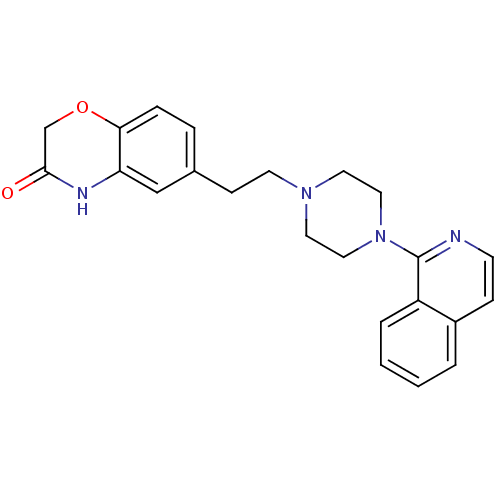
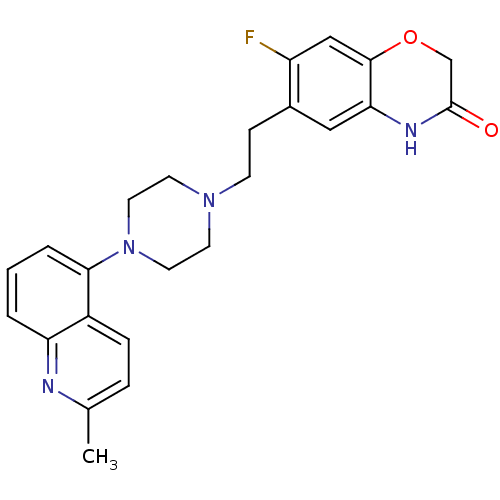
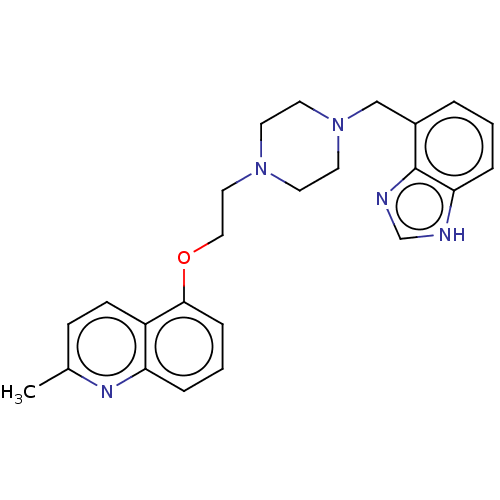
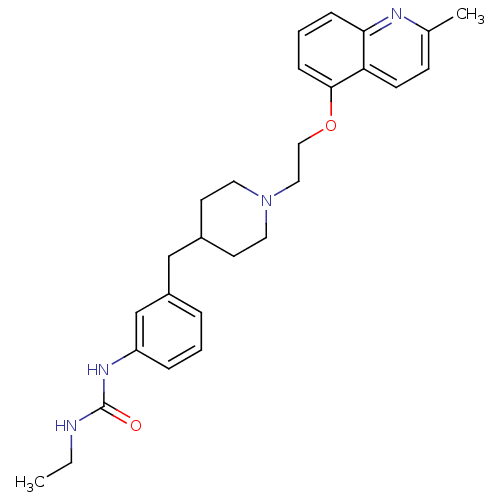
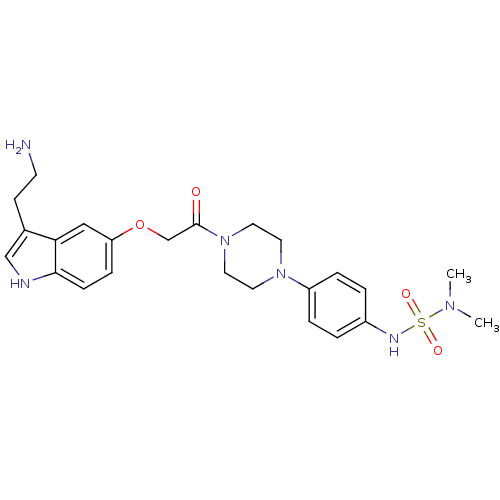
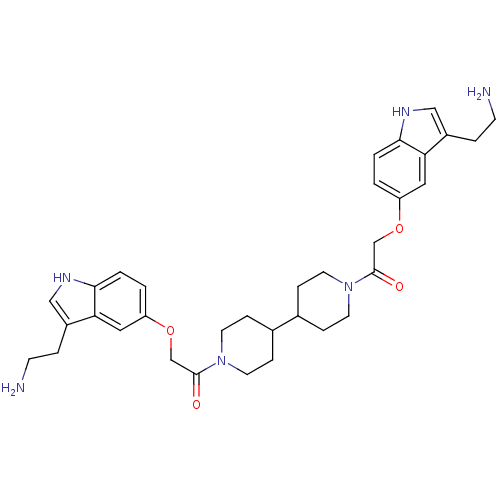
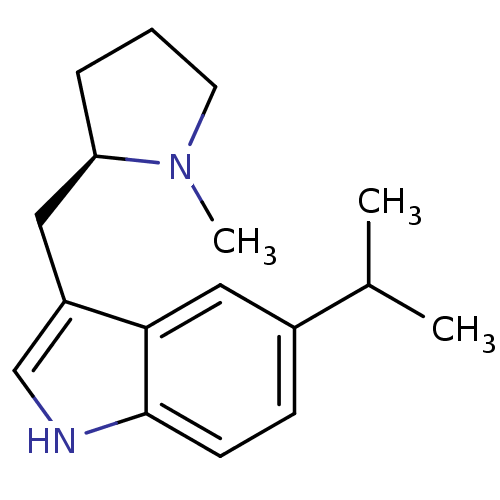
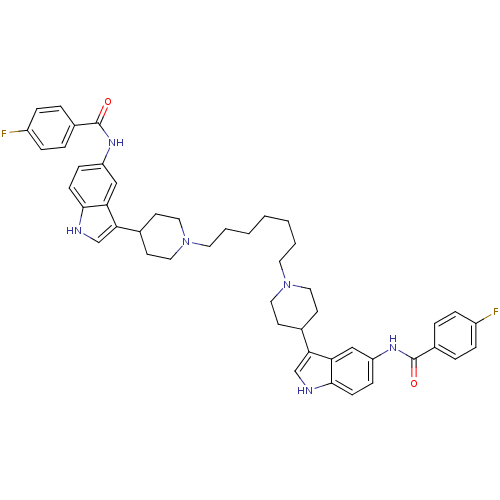
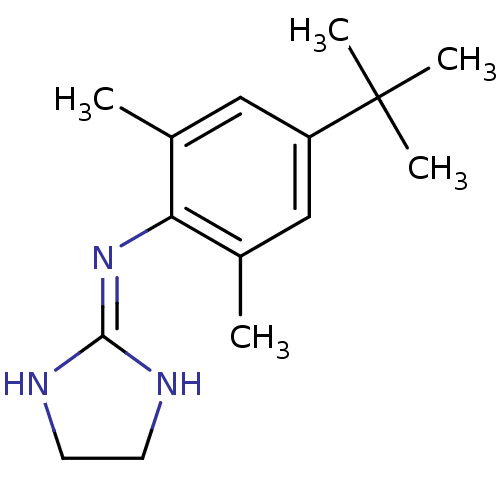
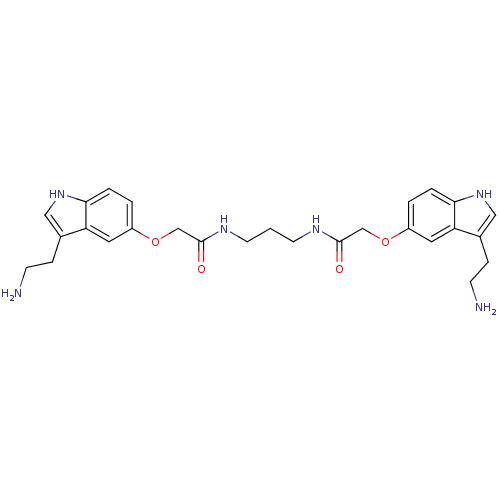
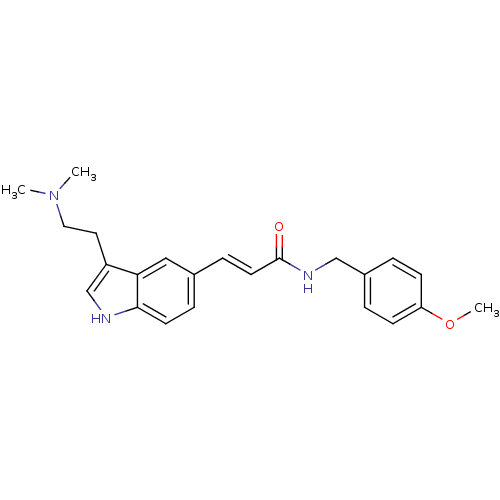
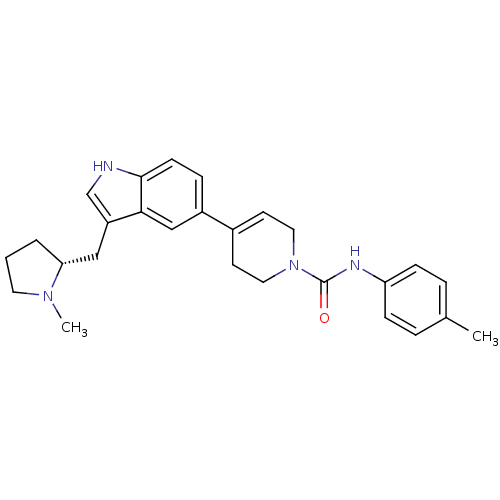
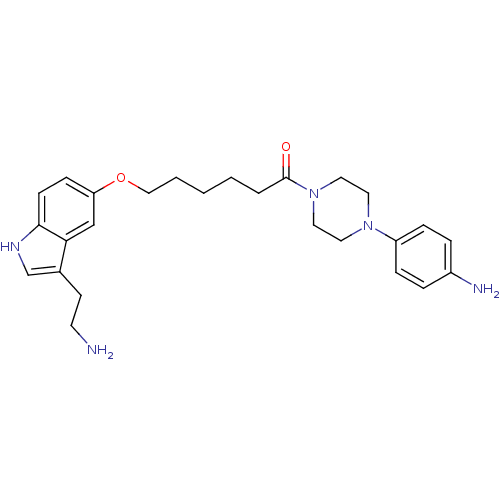
Report error Found 2232 Enz. Inhib. hit(s) with Target = '5-hydroxytryptamine receptor 1D'
Affinity DataKi: 0.0501nMAssay Description:Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HTMore data for this Ligand-Target Pair
Affinity DataKi: 0.0501nMAssay Description:Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.0794nMAssay Description:Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HTMore data for this Ligand-Target Pair
Affinity DataKi: 0.0794nMAssay Description:Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.0900nMAssay Description:Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HTMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.126nMAssay Description:Binding affinity against 5-hydroxytryptamine 1D receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 0.126nMAssay Description:Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.126nMAssay Description:Antagonist activity at human 5-HT1D receptor expressed in CHO cells assessed as inhibition of GTPgammaS binding by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.158nMAssay Description:Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HTMore data for this Ligand-Target Pair
Affinity DataKi: 0.158nMAssay Description:Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.158nMAssay Description:Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.158nMAssay Description:Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.180nMAssay Description:Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HTMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Displacement of [3H]5HT from human cloned 5HT1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.25nMAssay Description:Binding affinity for human 5-hydroxytryptamine 1D receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.251nMAssay Description:Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.251nMAssay Description:Antagonist activity at human 5HT1D assessed as GTPgammaS binding by scintillation proximity assay in presence of 5-HTMore data for this Ligand-Target Pair
Affinity DataKi: 0.251nMAssay Description:Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.251nMAssay Description:Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.251nMAssay Description:Displacement of [3H]5-HT from human 5-hydroxytryptamine 1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.251nMAssay Description:Displacement of [3H]5HT from human recombinant 5HT1D receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.260nMAssay Description:Binding affinity to recombinant human 5-hydroxytryptamine 1D receptor alphaMore data for this Ligand-Target Pair
Affinity DataKi: 0.270nMAssay Description:Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.290nMAssay Description:Binding affinity for human 5-hydroxytryptamine 1D receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Binding affinity for cloned human 5-hydroxytryptamine 1D receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Displacement of [3H]5-hydroxytryptamine from human cloned 5HT1D receptor expressed in CHOK1 cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Binding affinity towards human 5-hydroxytryptamine 1D receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Ability to inhibit the forskolin-stimulated c-AMP formation mediated by human 5-hydroxytryptamine 1D receptor in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor alpha expressed in CHO-K1 cells.More data for this Ligand-Target Pair
Affinity DataKi: 0.310nMAssay Description:Binding affinity towards cloned human 5-hydroxytryptamine 1D receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.310nMAssay Description:Binding affinity for cloned human 5-hydroxytryptamine 1D receptor betaMore data for this Ligand-Target Pair
Affinity DataKi: 0.316nMAssay Description:Displacement of [3H]5HT from human 5HT1D receptor expressed in CHO cellsMore data for this Ligand-Target Pair