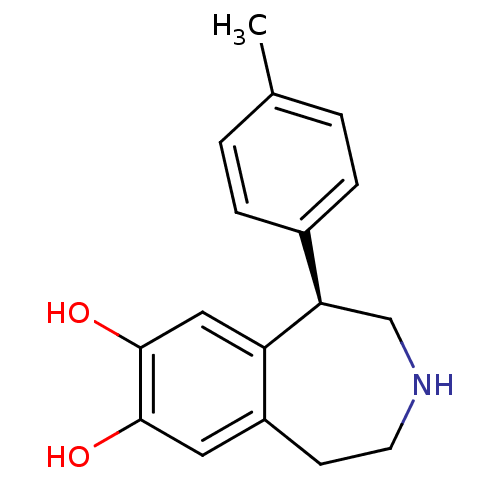
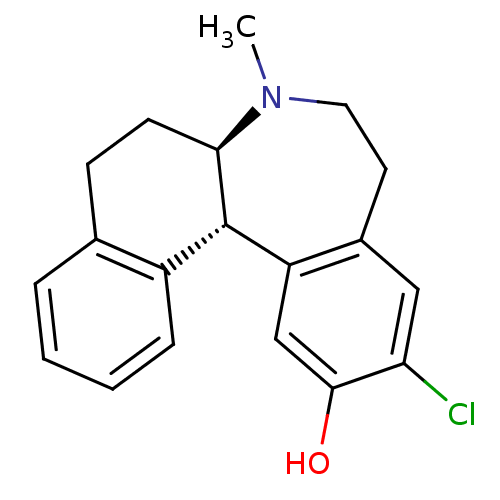
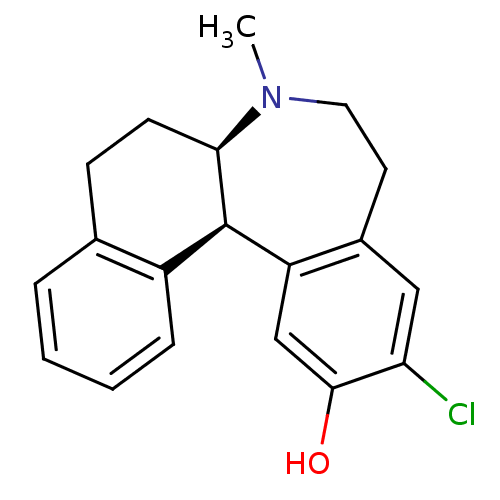
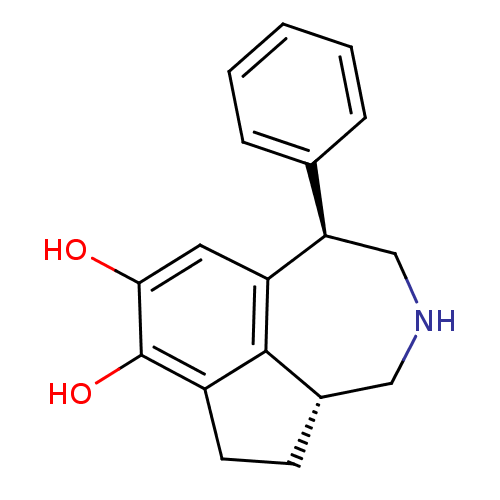
Report error Found 17 Enz. Inhib. hit(s) with all data for entry = 50035890
Affinity DataKi: 0.300nMAssay Description:Binding affinity to displace [3H]- SCH 23390 against Dopamine receptor D1More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Binding affinity to displace [3H]- SCH 23390 against Dopamine receptor D1More data for this Ligand-Target Pair
Affinity DataKi: 0.400nMAssay Description:Binding affinity to displace [3H]- SCH 23390 against Dopamine receptor D1More data for this Ligand-Target Pair
Affinity DataKi: 0.800nMAssay Description:Binding affinity to displace [3H]- SCH 23390 against Dopamine receptor D1More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Binding affinity to displace [3H]- SCH 23390 against Dopamine receptor D1More data for this Ligand-Target Pair
Affinity DataKi: 1.90nMAssay Description:Binding affinity to displace [3H]-SCH- 23390 against Dopamine receptor D1More data for this Ligand-Target Pair
Affinity DataKi: 5.10nMAssay Description:Binding affinity against Dopamine receptor D1 in rat radioligandMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Binding affinity to displace [3H]-SCH- 23390 against Dopamine receptor D1More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Binding affinity to displace [3H]- SCH 23390 against Dopamine receptor D1More data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Binding affinity against Dopamine receptor D1 in rat radioligandMore data for this Ligand-Target Pair
Affinity DataEC50: 80nMAssay Description:Effective concentration required to stimulate Adenylate cyclaseMore data for this Ligand-Target Pair
Affinity DataKi: 192nMAssay Description:Binding affinity to displace [3H]- SCH 23390 against Dopamine receptor D1More data for this Ligand-Target Pair
Affinity DataEC50: 210nMAssay Description:Effective concentration required to stimulate Adenylate cyclaseMore data for this Ligand-Target Pair
Affinity DataKi: 513nMAssay Description:Binding affinity to displace [3H]-SCH- 23390 against Dopamine receptor D1More data for this Ligand-Target Pair
Affinity DataKi: 531nMAssay Description:Binding affinity to displace [3H]-SCH- 23390 against Dopamine receptor D1More data for this Ligand-Target Pair
Affinity DataKi: 898nMAssay Description:Binding affinity to displace [3H]-SCH- 23390 against Dopamine receptor D1More data for this Ligand-Target Pair
Affinity DataKi: 1.22E+3nMAssay Description:Binding affinity against Dopamine receptor D1 in rat radioligandMore data for this Ligand-Target Pair
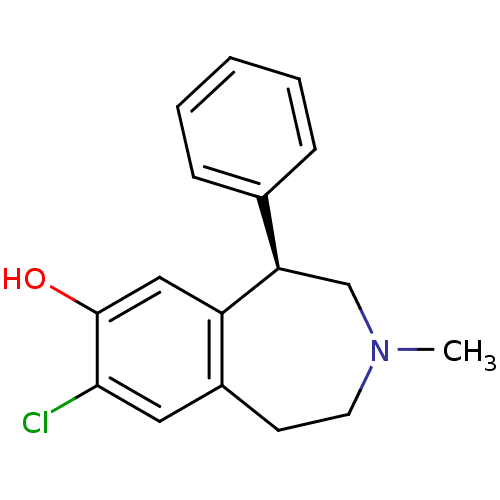
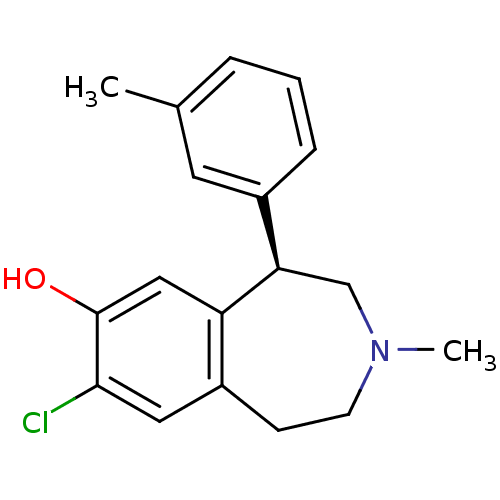
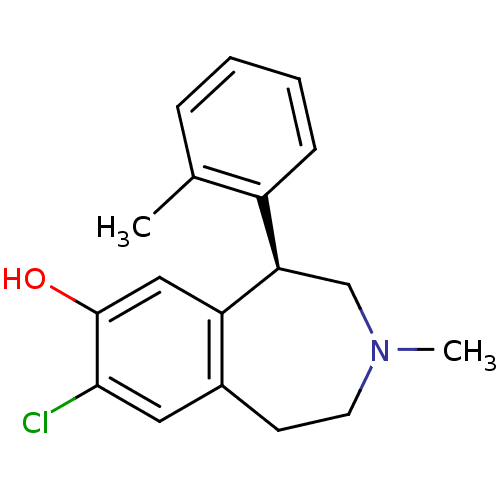
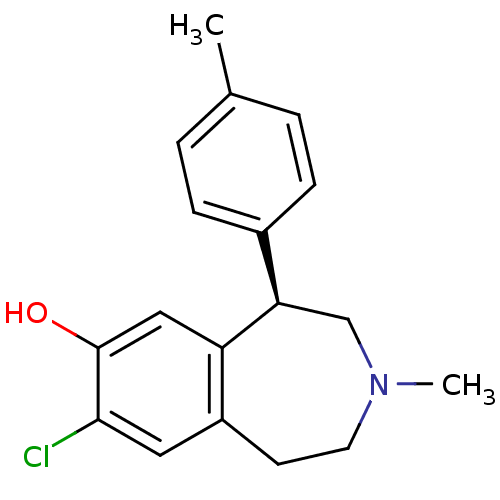
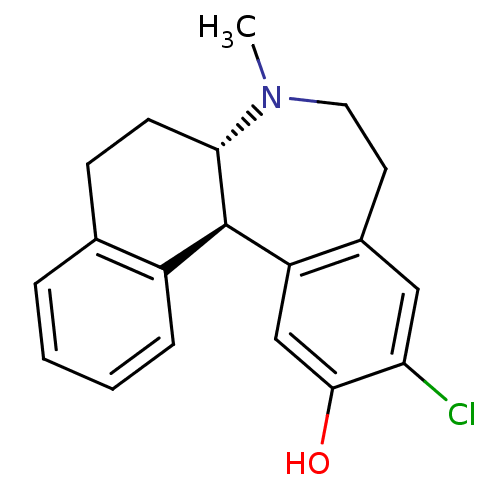
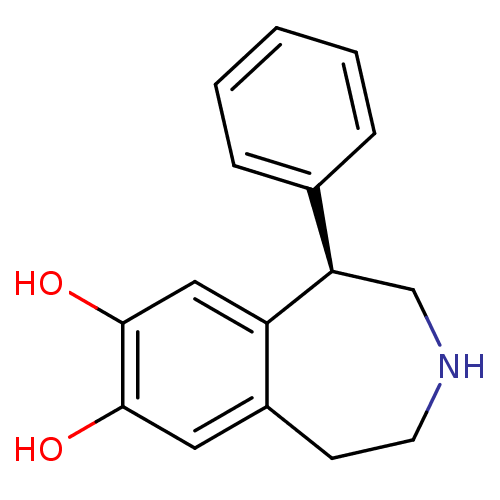
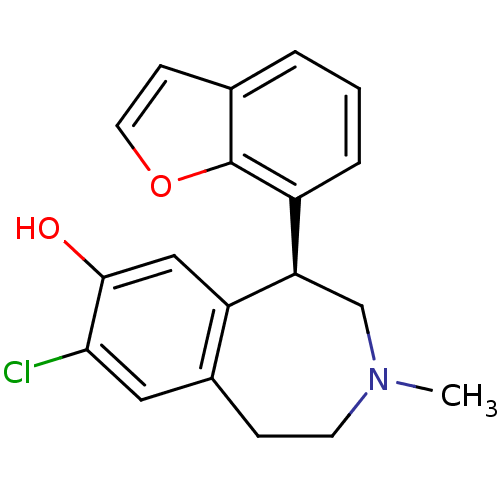
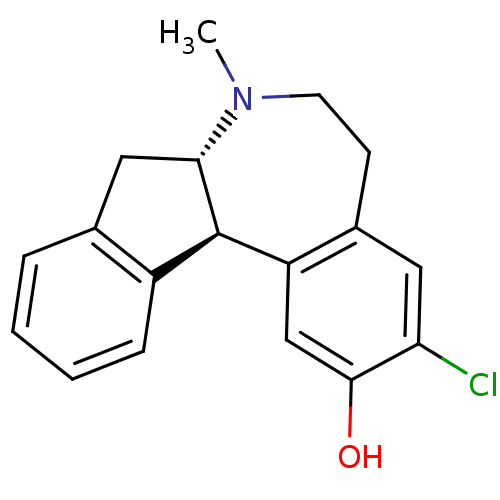
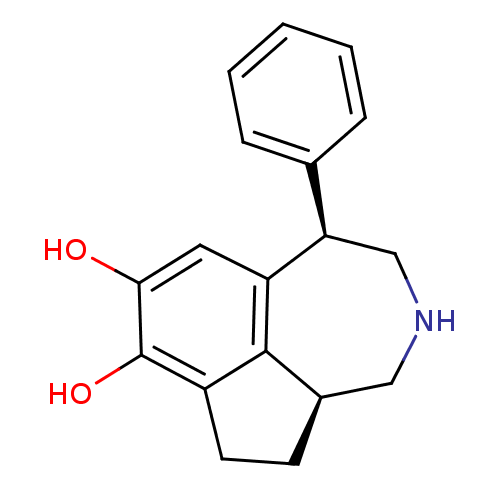
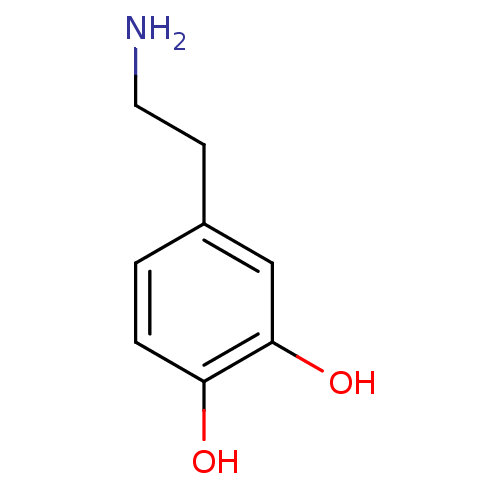
 3D Structure (crystal)
3D Structure (crystal)