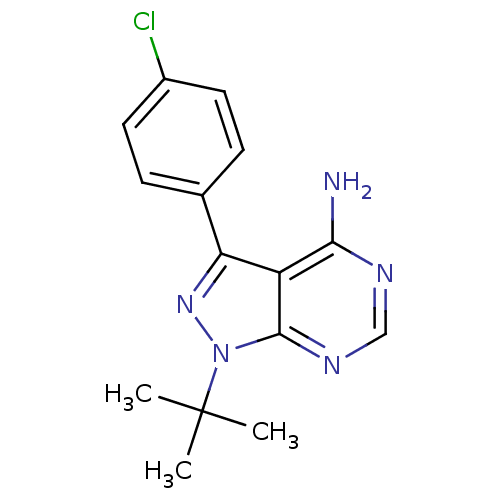
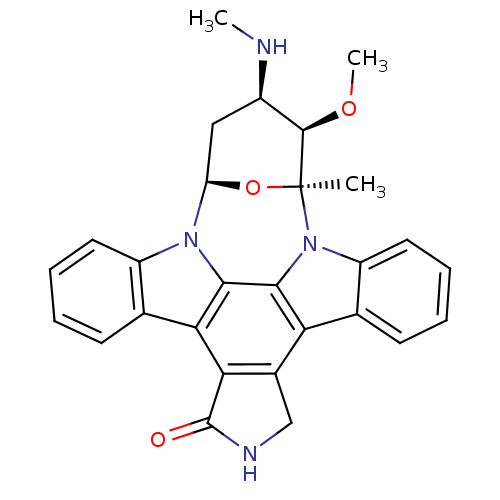
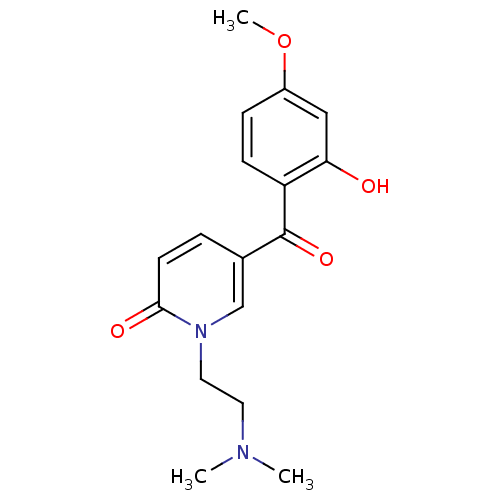
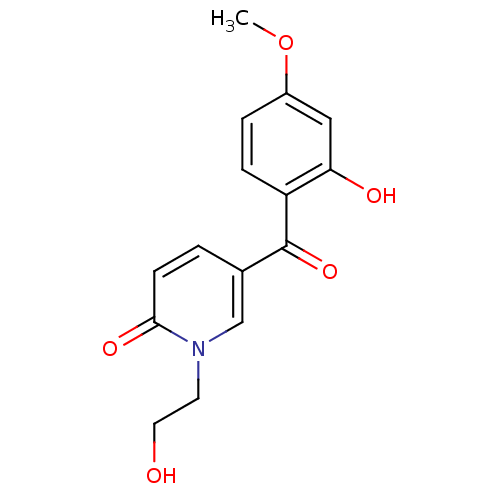
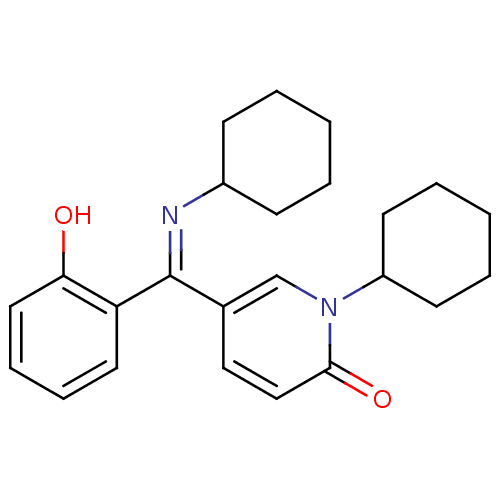
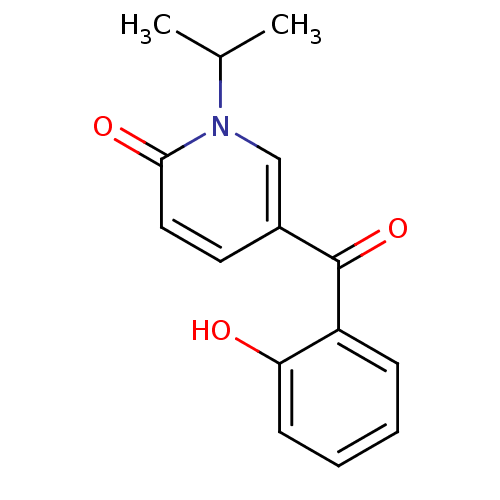
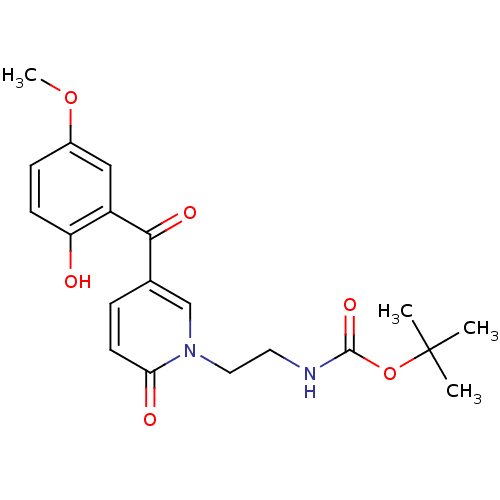
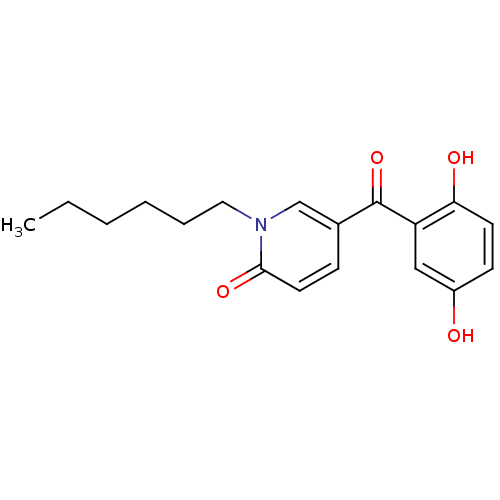
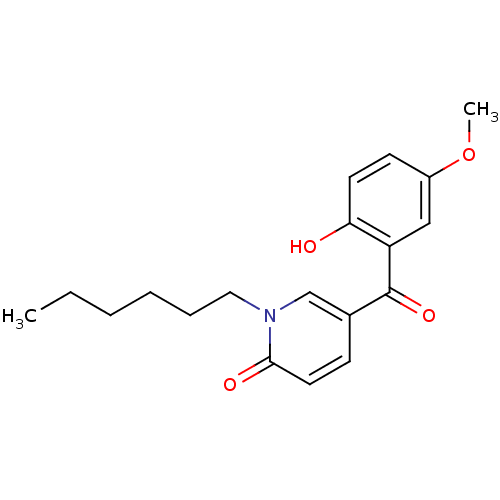
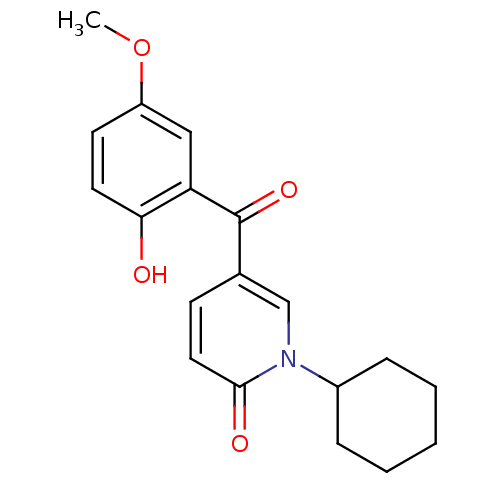
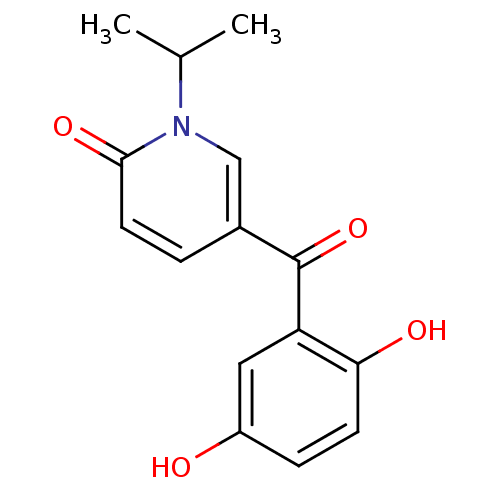
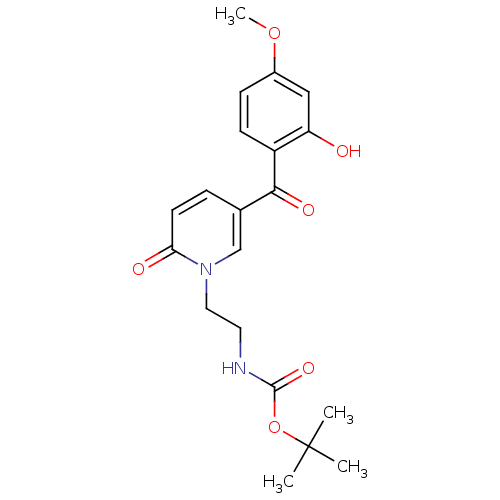
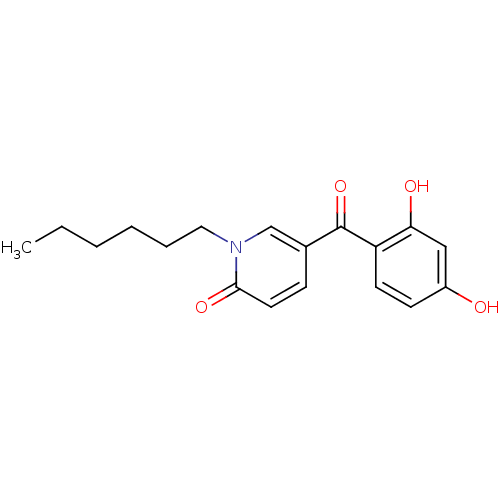
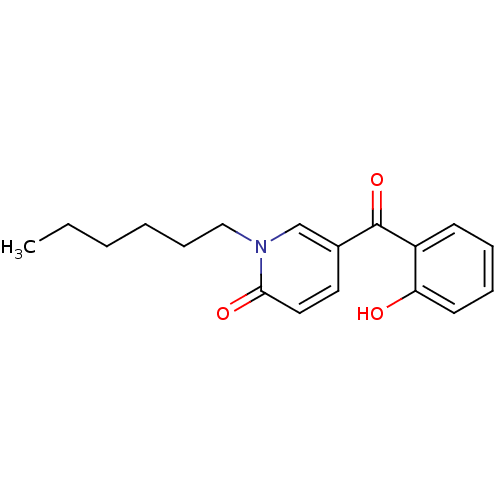
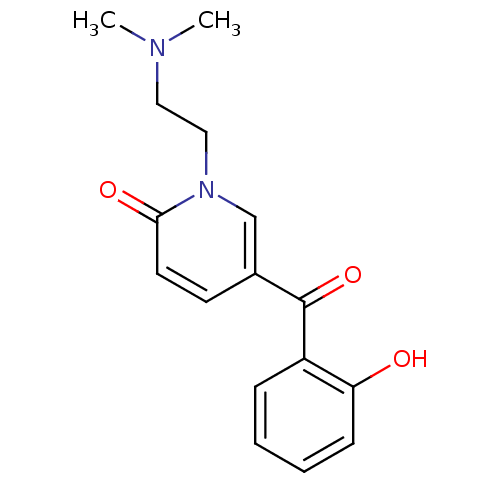
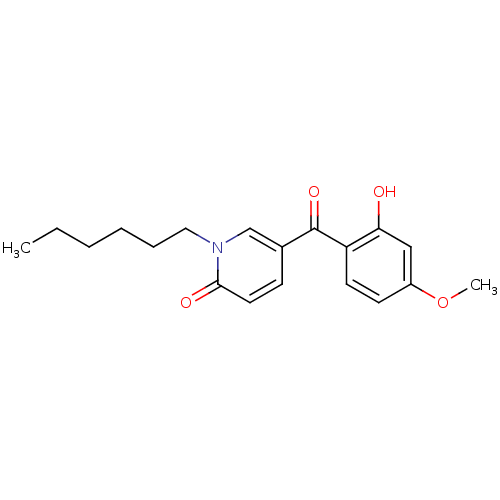
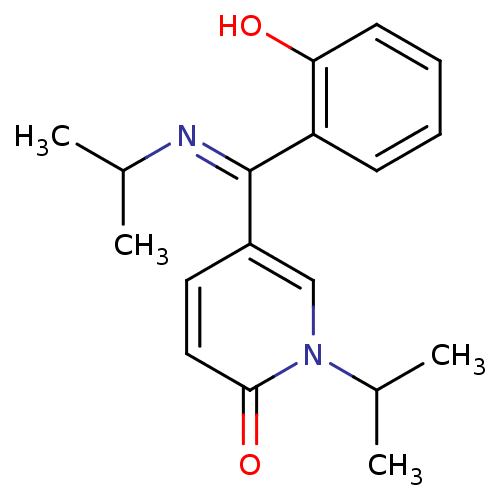
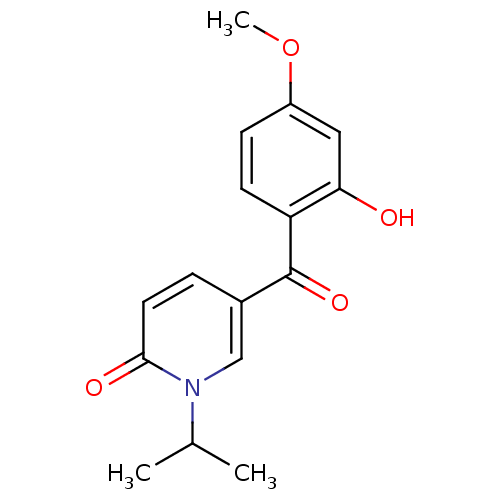
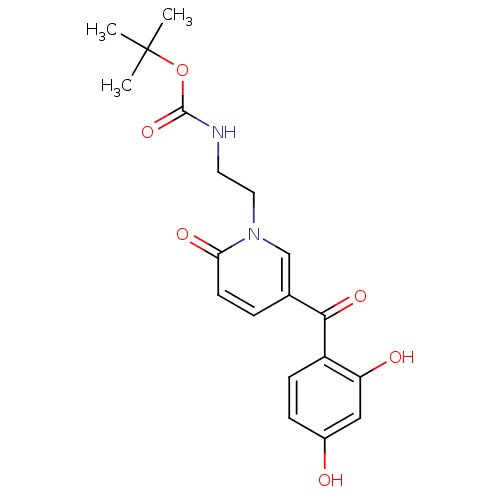
Report error Found 23 Enz. Inhib. hit(s) with all data for entry = 6128
Affinity DataIC50: 500nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 600nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 1.99E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 2.01E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 2.18E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 2.19E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 2.21E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 2.35E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 2.48E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 2.54E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 2.61E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 2.76E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 2.77E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 2.81E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 2.82E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 3.41E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 3.79E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 5.78E+4nMpH: 7.5 T: 2°CAssay Description:The kinase reaction was initiated with the incubation of the 2.5 µL of the reaction cocktail (0.7 nM of His6-Src kinase domain in kinase buffer)...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMpH: 7.0Assay Description:EGFR was incubated with 8 mM MOPS (pH 7.0), 0.2 mM EDTA, 10 mM MnCl2, 0.1 mg/mL poly(Glu, Tyr) 4:1. MAPK1 was incubated with 25 mM Tris (pH 7.5), 0.0...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMpH: 7.0Assay Description:EGFR was incubated with 8 mM MOPS (pH 7.0), 0.2 mM EDTA, 10 mM MnCl2, 0.1 mg/mL poly(Glu, Tyr) 4:1. MAPK1 was incubated with 25 mM Tris (pH 7.5), 0.0...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMpH: 7.0Assay Description:EGFR was incubated with 8 mM MOPS (pH 7.0), 0.2 mM EDTA, 10 mM MnCl2, 0.1 mg/mL poly(Glu, Tyr) 4:1. MAPK1 was incubated with 25 mM Tris (pH 7.5), 0.0...More data for this Ligand-Target Pair