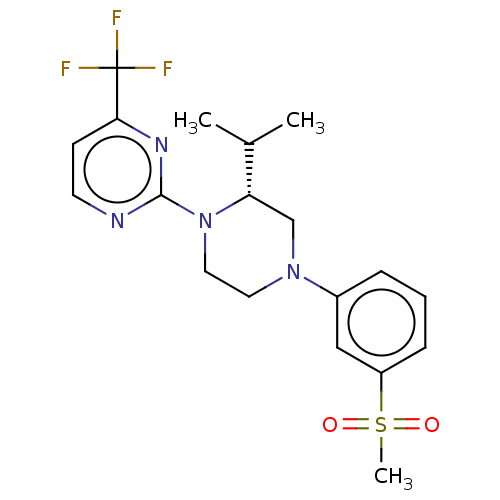
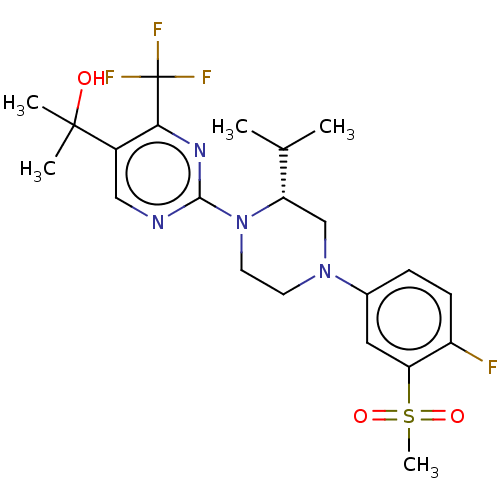
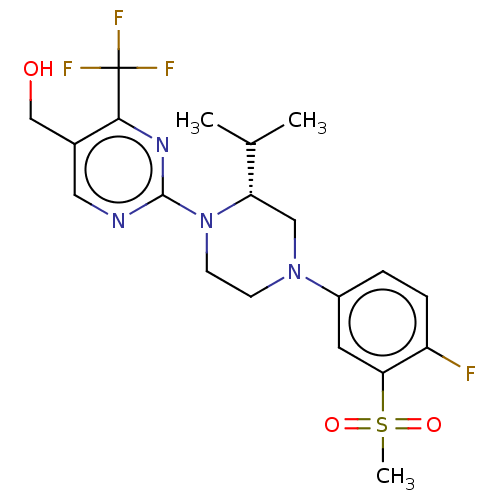
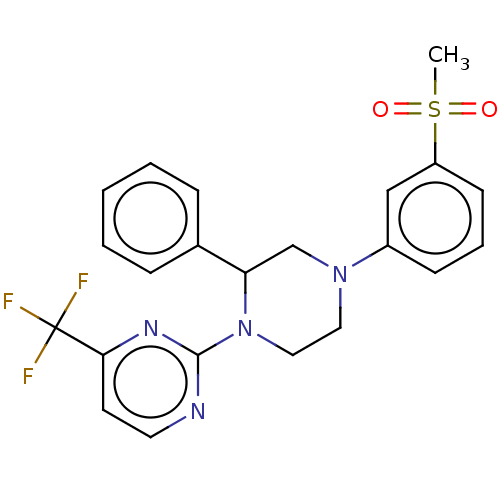
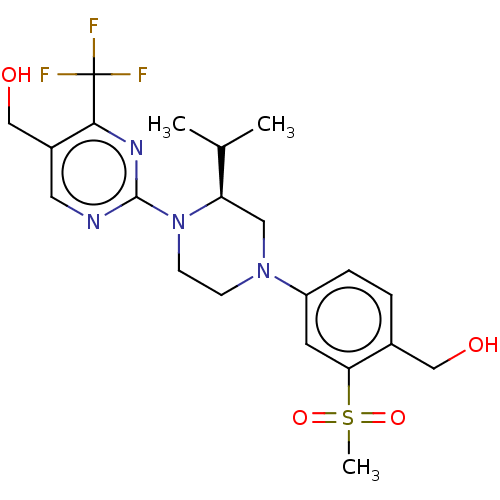
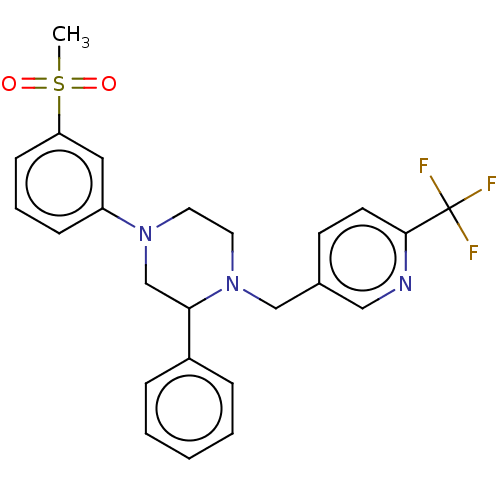
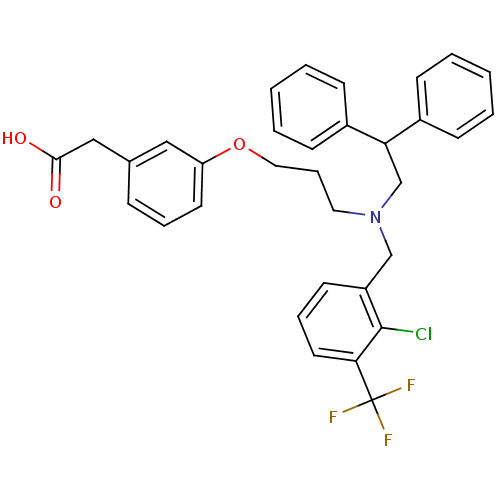
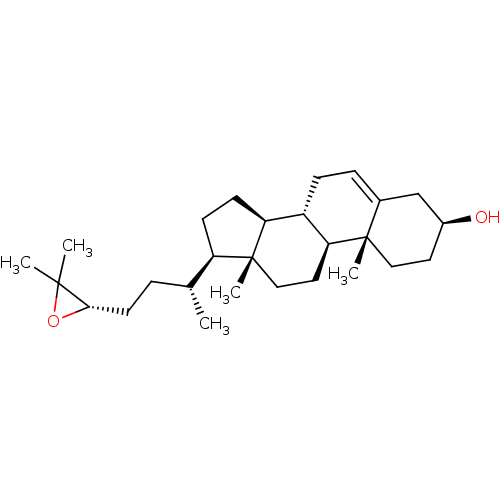
Affinity DataKi: 1nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 81nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 94nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 94nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 146nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 157nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.07E+3nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.27E+3nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.28E+3nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.20E+3nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: >2.50E+3nMAssay Description:Displacement of [3H]TO901317 from LXRbeta ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: >3.30E+3nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: >3.30E+3nMAssay Description:Displacement of [3H]TO901317 from LXRalpha ligand binding domain (unknown origin) after 30 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Binding to human LXRbeta by radioligand displacement assayMore data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Binding affinity to human LXRalpha by radioligand displacement assayMore data for this Ligand-Target Pair
Affinity DataIC50: 610nMAssay Description:Inhibition of recombinant CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of CYP2C9 in human liver microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 6.60E+3nMAssay Description:Inhibition of CYP3A4 in human liver microsomesMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Vitae Pharmaceuticals
Curated by ChEMBL
Vitae Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of PXR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of RORalpha receptor (unknown origin)More data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Vitae Pharmaceuticals
Curated by ChEMBL
Vitae Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of RXR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Mineralocorticoid receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.94E+4nMAssay Description:Inhibition of recombinant CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of recombinant CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 1.13E+3nMAssay Description:Agonist activity at human LXRalpha by Gal4 assayMore data for this Ligand-Target Pair
Affinity DataEC50: 390nMAssay Description:Agonist activity at human LXRbeta by Gal4 assayMore data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+4nMAssay Description:Binding affinity to human LXRalphaMore data for this Ligand-Target Pair
Affinity DataEC50: 22nMAssay Description:Binding affinity to human LXRalphaMore data for this Ligand-Target Pair
Affinity DataEC50: 19nMAssay Description:Binding affinity to human LXRalphaMore data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+4nMAssay Description:Binding to human LXRbetaMore data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Binding to human LXRbetaMore data for this Ligand-Target Pair
Affinity DataEC50: 1nMAssay Description:Binding to human LXRbetaMore data for this Ligand-Target Pair
Affinity DataEC50: 117nMAssay Description:Agonist activity at human LXRalpha coexpressed in African green monkey CV1 cells by Gal4 responsive reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 205nMAssay Description:Agonist activity at human LXRalpha coexpressed in African green monkey CV1 cells by Gal4 responsive reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 168nMAssay Description:Agonist activity at human LXRbeta coexpressed in African green monkey CV1 cells by Gal4 responsive reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 30nMAssay Description:Agonist activity at human LXRbeta coexpressed in African green monkey CV1 cells by Gal4 responsive reporter gene assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha/LXR-beta(Homo sapiens (Human))
Vitae Pharmaceuticals
Curated by ChEMBL
Vitae Pharmaceuticals
Curated by ChEMBL
Affinity DataEC50: 4nMAssay Description:Agonist activity at LXR in human THP1 cells assessed as upregulation of ABCA1 gene expression after 24 hrs by RT-PCR analysisMore data for this Ligand-Target Pair

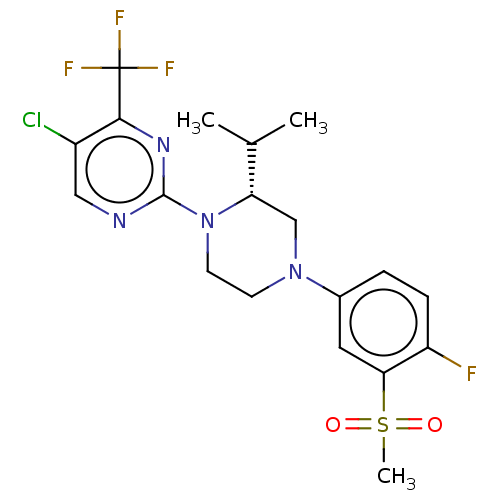
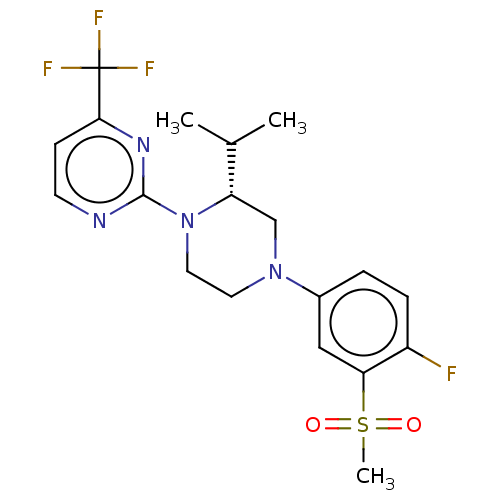
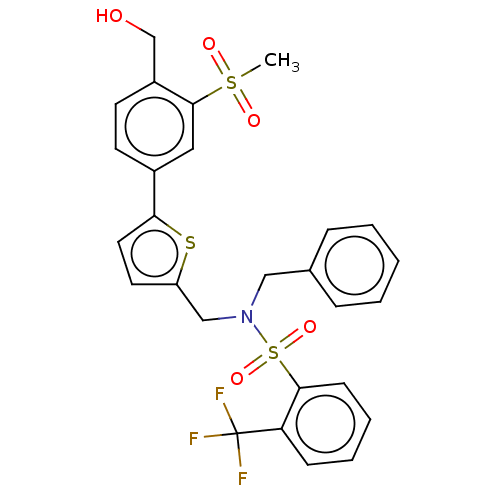
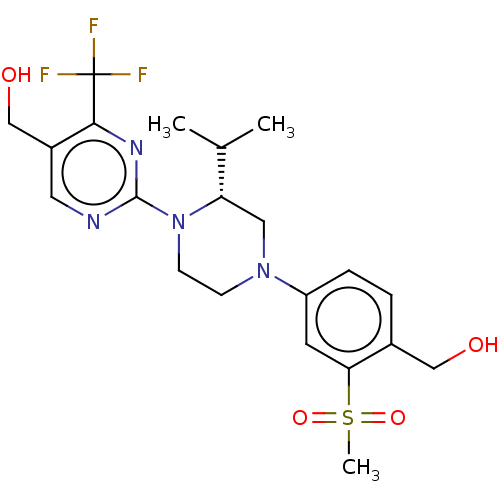
 3D Structure (crystal)
3D Structure (crystal)