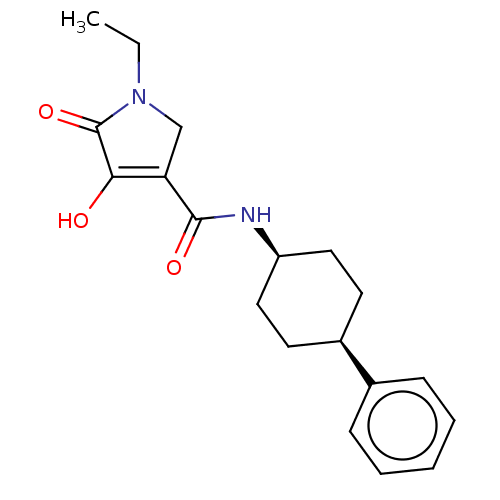
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Endothelial lipase
Ligand
BDBM50506732
Substrate
n/a
Meas. Tech.
ChEMBL_1827144 (CHEMBL4327018)
IC50
82±n/a nM
Citation
 Hu, CH; Wang, TC; Qiao, JX; Haque, L; Chen, AYA; Taylor, DS; Ying, X; Onorato, JM; Galella, M; Shen, H; Huang, CS; Toussaint, N; Li, YX; Abell, L; Adam, LP; Gordon, D; Wexler, RR; Finlay, HJ Discovery and synthesis of tetrahydropyrimidinedione-4-carboxamides as endothelial lipase inhibitors. Bioorg Med Chem Lett 28:3721-3725 (2018) [PubMed] Article
Hu, CH; Wang, TC; Qiao, JX; Haque, L; Chen, AYA; Taylor, DS; Ying, X; Onorato, JM; Galella, M; Shen, H; Huang, CS; Toussaint, N; Li, YX; Abell, L; Adam, LP; Gordon, D; Wexler, RR; Finlay, HJ Discovery and synthesis of tetrahydropyrimidinedione-4-carboxamides as endothelial lipase inhibitors. Bioorg Med Chem Lett 28:3721-3725 (2018) [PubMed] Article More Info.:
Target
Name:
Endothelial lipase
Synonyms:
EDL | EL | Endothelial cell-derived lipase | LIPE_HUMAN | LIPG
Type:
Protein
Mol. Mass.:
56805.62
Organism:
Homo sapiens (Human)
Description:
Q9Y5X9
Residue:
500
Sequence:
MSNSVPLLCFWSLCYCFAAGSPVPFGPEGRLEDKLHKPKATQTEVKPSVRFNLRTSKDPEHEGCYLSVGHSQPLEDCSFNMTAKTFFIIHGWTMSGIFENWLHKLVSALHTREKDANVVVVDWLPLAHQLYTDAVNNTRVVGHSIARMLDWLQEKDDFSLGNVHLIGYSLGAHVAGYAGNFVKGTVGRITGLDPAGPMFEGADIHKRLSPDDADFVDVLHTYTRSFGLSIGIQMPVGHIDIYPNGGDFQPGCGLNDVLGSIAYGTITEVVKCEHERAVHLFVDSLVNQDKPSFAFQCTDSNRFKKGICLSCRKNRCNSIGYNAKKMRNKRNSKMYLKTRAGMPFRVYHYQMKIHVFSYKNMGEIEPTFYVTLYGTNADSQTLPLEIVERIEQNATNTFLVYTEEDLGDLLKIQLTWEGASQSWYNLWKEFRSYLSQPRNPGRELNIRRIRVKSGETQRKLTFCTEDPENTSISPGRELWFRKCRDGWRMKNETSPTVELP
Inhibitor
Name:
BDBM50506732
Synonyms:
CHEMBL4592858
Type:
Small organic molecule
Emp. Form.:
C19H24N2O3
Mol. Mass.:
328.4055
SMILES:
CCN1CC(C(=O)N[C@H]2CC[C@H](CC2)c2ccccc2)=C(O)C1=O |r,wU:8.7,11.14,t:21,(33.21,-13.77,;34.11,-12.51,;33.48,-11.11,;31.97,-10.8,;31.8,-9.27,;30.46,-8.5,;30.46,-6.96,;29.13,-9.28,;27.8,-8.51,;26.47,-9.29,;25.14,-8.52,;25.13,-6.98,;26.47,-6.21,;27.8,-6.97,;23.8,-6.21,;23.81,-4.67,;22.47,-3.9,;21.14,-4.67,;21.14,-6.21,;22.48,-6.98,;33.21,-8.64,;33.52,-7.13,;34.24,-9.77,;35.78,-9.61,)|