Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50565837
Substrate
n/a
Meas. Tech.
ChEMBL_2093987 (CHEMBL4775250)
IC50
>40000±n/a nM
Citation
 Sun, LQ; Mull, E; D'Andrea, S; Zheng, B; Hiebert, S; Gillis, E; Bowsher, M; Kandhasamy, S; Baratam, VR; Puttaswamy, S; Pulicharla, N; Vishwakrishnan, S; Reddy, S; Trivedi, R; Sinha, S; Sivaprasad, S; Rao, A; Desai, S; Ghosh, K; Anumula, R; Kumar, A; Rajamani, R; Wang, YK; Fang, H; Mathur, A; Rampulla, R; Zvyaga, TA; Mosure, K; Jenkins, S; Falk, P; Tagore, DM; Chen, C; Rendunchintala, K; Loy, J; Meanwell, NA; McPhee, F; Scola, PM Discovery of BMS-986144, a Third-Generation, Pan-Genotype NS3/4A Protease Inhibitor for the Treatment of Hepatitis C Virus Infection. J Med Chem 63:14740-14760 (2020) [PubMed] Article
Sun, LQ; Mull, E; D'Andrea, S; Zheng, B; Hiebert, S; Gillis, E; Bowsher, M; Kandhasamy, S; Baratam, VR; Puttaswamy, S; Pulicharla, N; Vishwakrishnan, S; Reddy, S; Trivedi, R; Sinha, S; Sivaprasad, S; Rao, A; Desai, S; Ghosh, K; Anumula, R; Kumar, A; Rajamani, R; Wang, YK; Fang, H; Mathur, A; Rampulla, R; Zvyaga, TA; Mosure, K; Jenkins, S; Falk, P; Tagore, DM; Chen, C; Rendunchintala, K; Loy, J; Meanwell, NA; McPhee, F; Scola, PM Discovery of BMS-986144, a Third-Generation, Pan-Genotype NS3/4A Protease Inhibitor for the Treatment of Hepatitis C Virus Infection. J Med Chem 63:14740-14760 (2020) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Human
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
Inhibitor
Name:
BDBM50565837
Synonyms:
CHEMBL4787795
Type:
Small organic molecule
Emp. Form.:
C40H51F4N5O9S
Mol. Mass.:
853.92
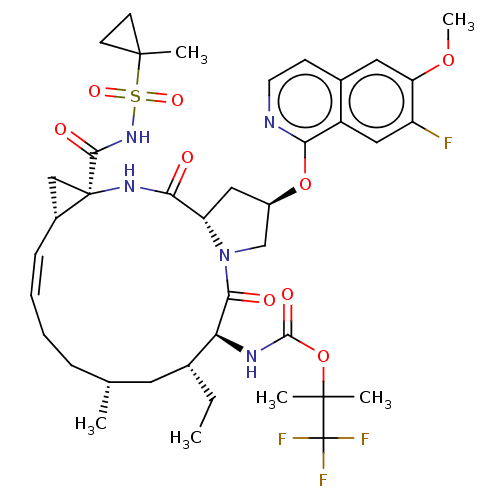
SMILES:
[H][C@@]12C[C@]1(NC(=O)[C@]1([H])C[C@H](CN1C(=O)[C@@H](NC(=O)OC(C)(C)C(F)(F)F)[C@H](CC)C[C@H](C)CC\C=C/2)Oc1nccc2cc(OC)c(F)cc12)C(=O)NS(=O)(=O)C1(C)CC1 |r,c:37|