Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Monoglyceride lipase
Ligand
BDBM50566993
Substrate
n/a
Meas. Tech.
ChEMBL_2102182 (CHEMBL4810578)
IC50
38±n/a nM
Citation
 Ikeda, S; Sugiyama, H; Tokuhara, H; Murakami, M; Nakamura, M; Oguro, Y; Aida, J; Morishita, N; Sogabe, S; Dougan, DR; Gay, SC; Qin, L; Arimura, N; Takahashi, Y; Sasaki, M; Kamada, Y; Aoyama, K; Kimoto, K; Kamata, M Design and Synthesis of Novel Spiro Derivatives as Potent and Reversible Monoacylglycerol Lipase (MAGL) Inhibitors: Bioisosteric Transformation from 3-Oxo-3,4-dihydro-2 J Med Chem 64:11014-11044 (2021) [PubMed] Article
Ikeda, S; Sugiyama, H; Tokuhara, H; Murakami, M; Nakamura, M; Oguro, Y; Aida, J; Morishita, N; Sogabe, S; Dougan, DR; Gay, SC; Qin, L; Arimura, N; Takahashi, Y; Sasaki, M; Kamada, Y; Aoyama, K; Kimoto, K; Kamata, M Design and Synthesis of Novel Spiro Derivatives as Potent and Reversible Monoacylglycerol Lipase (MAGL) Inhibitors: Bioisosteric Transformation from 3-Oxo-3,4-dihydro-2 J Med Chem 64:11014-11044 (2021) [PubMed] Article More Info.:
Target
Name:
Monoglyceride lipase
Synonyms:
HU-K5 | Lysophospholipase homolog | Lysophospholipase-like | MAGL | MGL | MGLL | MGLL_HUMAN
Type:
Hydrolase
Mol. Mass.:
33264.56
Organism:
Homo sapiens (Human)
Description:
Human recombinant MGL (Cayman Chemical, cat# 10008354).
Residue:
303
Sequence:
MPEESSPRRTPQSIPYQDLPHLVNADGQYLFCRYWKPTGTPKALIFVSHGAGEHSGRYEELARMLMGLDLLVFAHDHVGHGQSEGERMVVSDFHVFVRDVLQHVDSMQKDYPGLPVFLLGHSMGGAIAILTAAERPGHFAGMVLISPLVLANPESATTFKVLAAKVLNLVLPNLSLGPIDSSVLSRNKTEVDIYNSDPLICRAGLKVCFGIQLLNAVSRVERALPKLTVPFLLLQGSADRLCDSKGAYLLMELAKSQDKTLKIYEGAYHVLHKELPEVTNSVFHEINMWVSQRTATAGTASPP
Inhibitor
Name:
BDBM50566993
Synonyms:
CHEMBL4874410
Type:
Small organic molecule
Emp. Form.:
C18H20ClFN2O4
Mol. Mass.:
382.814
SMILES:
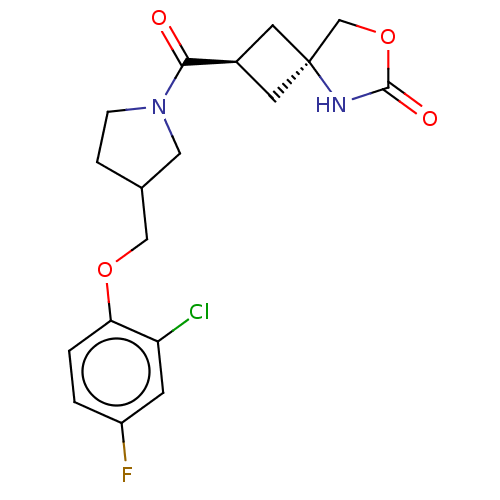
Fc1ccc(OCC2CCN(C2)C(=O)[C@H]2C[C@@]3(C2)COC(=O)N3)c(Cl)c1 |r,wD:14.14,16.24,(44.73,-23.76,;46.06,-22.99,;47.4,-23.76,;48.73,-22.99,;48.73,-21.46,;50.07,-20.7,;51.4,-21.47,;52.73,-20.7,;53.5,-22.04,;55.01,-21.72,;55.17,-20.18,;53.76,-19.56,;56.5,-19.41,;56.5,-17.87,;57.83,-20.18,;58.22,-21.66,;59.71,-21.26,;59.31,-19.78,;59.7,-22.8,;61.16,-23.29,;62.08,-22.05,;63.62,-22.05,;61.18,-20.8,;47.4,-20.68,;47.41,-19.14,;46.07,-21.45,)|