Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Carboxylic ester hydrolase
Ligand
BDBM10813
Substrate
BDBM8978
Meas. Tech.
Cholinesterase Inhibition Assay
IC50
210±n/a nM
Citation
 Sterling, J; Herzig, Y; Goren, T; Finkelstein, N; Lerner, D; Goldenberg, W; Miskolczi, I; Molnar, S; Rantal, F; Tamas, T; Toth, G; Zagyva, A; Zekany, A; Finberg, J; Lavian, G; Gross, A; Friedman, R; Razin, M; Huang, W; Krais, B; Chorev, M; Youdim, MB; Weinstock, M Novel dual inhibitors of AChE and MAO derived from hydroxy aminoindan and phenethylamine as potential treatment for Alzheimer's disease. J Med Chem 45:5260-79 (2002) [PubMed] Article
Sterling, J; Herzig, Y; Goren, T; Finkelstein, N; Lerner, D; Goldenberg, W; Miskolczi, I; Molnar, S; Rantal, F; Tamas, T; Toth, G; Zagyva, A; Zekany, A; Finberg, J; Lavian, G; Gross, A; Friedman, R; Razin, M; Huang, W; Krais, B; Chorev, M; Youdim, MB; Weinstock, M Novel dual inhibitors of AChE and MAO derived from hydroxy aminoindan and phenethylamine as potential treatment for Alzheimer's disease. J Med Chem 45:5260-79 (2002) [PubMed] Article More Info.:
Target
Name:
Carboxylic ester hydrolase
Synonyms:
BuChE | Butyrlcholinesterase (BuChE) | Butyrylcholine esterase | Butyrylcholinesterase | Butyrylcholinesterase (BChE) | Butyrylcholinesterase (BuChE) | Butyrylcholinesterase (EqBuChE) | Carboxylic ester hydrolase | butyrylcholinesterase precursor
Type:
Protein
Mol. Mass.:
68842.83
Organism:
Equus caballus (Horse)
Description:
Q9N1N9
Residue:
602
Sequence:
MQSWGTIICIRILLRFLLLWVLIGNSHTEEDIIITTKNGKVRGMNLPVLGGTVTAFLGIPYAQPPLGRLRFKKPQSLTKWSNIWNATKYANSCYQNTDQSFPGFLGSEMWNPNTELSEDCLYLNVWIPAPKPKNATVMIWIYGGGFQTGTSSLPVYDGKFLARVERVIVVSMNYRVGALGFLALSENPEAPGNMGLFDQQLALQWVQKNIAAFGGNPRSVTLFGESAGAASVSLHLLSPRSQPLFTRAILQSGSSNAPWAVTSLYEARNRTLTLAKRMGCSRDNETEMIKCLRDKDPQEILLNEVFVVPYDTLLSVNFGPTVDGDFLTDMPDTLLQLGQFKRTQILVGVNKDEGTAFLVYGAPGFSKDNNSIITRKEFQEGLKIFFPRVSEFGRESILFHYMDWLDDQRAENYREALDDVVGDYNIICPALEFTKKFSELGNDAFFYYFEHRSTKLPWPEWMGVMHGYEIEFVFGLPLERRVNYTKAEEILSRSIMKRWANFAKYGNPNGTQSNSTRWPVFKSTEQKYLTLNTESPKVYTKLRAQQCRFWTLFFPKVLELTGNIDEAEREWKAGFHRWNNYMMDWKNQFNDYTSKKESCSDF
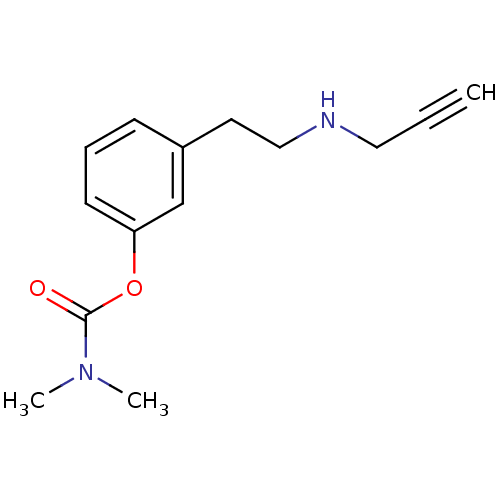
Inhibitor
Name:
BDBM10813
Synonyms:
3-[2-(prop-2-yn-1-ylamino)ethyl]phenyl N,N-dimethylcarbamate | Phenethylamine deriv. 50a
Type:
Small organic molecule
Emp. Form.:
C14H18N2O2
Mol. Mass.:
246.3049
SMILES:
CN(C)C(=O)Oc1cccc(CCNCC#C)c1
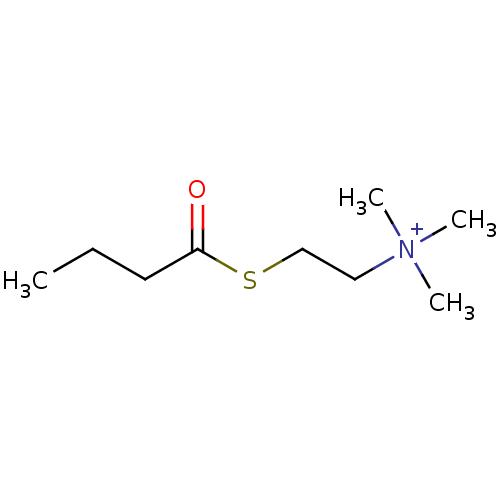
Substrate
Name:
BDBM8978
Synonyms:
(Propylcarbonylthioethyl)trimethylammonium iodide | CHEMBL139908 | CHEMBL148530 | [2-(butanoylsulfanyl)ethyl]trimethylazanium iodide | butyrylthiocholine | butyrylthiocholine chloride
Type:
Small organic molecule
Emp. Form.:
C9H20NOS
Mol. Mass.:
190.326
SMILES:
CCCC(=O)SCC[N+](C)(C)C