Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Prothrombin
Ligand
BDBM14131
Substrate
BDBM14064
Meas. Tech.
Enzyme Inhibition Assay
Ki
110±n/a nM
IC50
1900±n/a nM
Citation
 Costanzo, MJ; Almond, HR; Hecker, LR; Schott, MR; Yabut, SC; Zhang, HC; Andrade-Gordon, P; Corcoran, TW; Giardino, EC; Kauffman, JA; Lewis, JM; de Garavilla, L; Haertlein, BJ; Maryanoff, BE In-depth study of tripeptide-based alpha-ketoheterocycles as inhibitors of thrombin. Effective utilization of the S1' subsite and its implications to structure-based drug design. J Med Chem 48:1984-2008 (2005) [PubMed] Article
Costanzo, MJ; Almond, HR; Hecker, LR; Schott, MR; Yabut, SC; Zhang, HC; Andrade-Gordon, P; Corcoran, TW; Giardino, EC; Kauffman, JA; Lewis, JM; de Garavilla, L; Haertlein, BJ; Maryanoff, BE In-depth study of tripeptide-based alpha-ketoheterocycles as inhibitors of thrombin. Effective utilization of the S1' subsite and its implications to structure-based drug design. J Med Chem 48:1984-2008 (2005) [PubMed] Article More Info.:
Target
Name:
Prothrombin
Synonyms:
Activation peptide fragment 1 | Activation peptide fragment 2 | Coagulation factor II | F2 | Prothrombin precursor | THRB_HUMAN | Thrombin heavy chain | Thrombin light chain
Type:
Protein
Mol. Mass.:
70029.57
Organism:
Homo sapiens (Human)
Description:
P00734
Residue:
622
Sequence:
MAHVRGLQLPGCLALAALCSLVHSQHVFLAPQQARSLLQRVRRANTFLEEVRKGNLERECVEETCSYEEAFEALESSTATDVFWAKYTACETARTPRDKLAACLEGNCAEGLGTNYRGHVNITRSGIECQLWRSRYPHKPEINSTTHPGADLQENFCRNPDSSTTGPWCYTTDPTVRRQECSIPVCGQDQVTVAMTPRSEGSSVNLSPPLEQCVPDRGQQYQGRLAVTTHGLPCLAWASAQAKALSKHQDFNSAVQLVENFCRNPDGDEEGVWCYVAGKPGDFGYCDLNYCEEAVEEETGDGLDEDSDRAIEGRTATSEYQTFFNPRTFGSGEADCGLRPLFEKKSLEDKTERELLESYIDGRIVEGSDAEIGMSPWQVMLFRKSPQELLCGASLISDRWVLTAAHCLLYPPWDKNFTENDLLVRIGKHSRTRYERNIEKISMLEKIYIHPRYNWRENLDRDIALMKLKKPVAFSDYIHPVCLPDRETAASLLQAGYKGRVTGWGNLKETWTANVGKGQPSVLQVVNLPIVERPVCKDSTRIRITDNMFCAGYKPDEGKRGDACEGDSGGPFVMKSPFNNRWYQMGIVSWGEGCDRDGKYGFYTHVFRLKKWIQKVIDQFGE
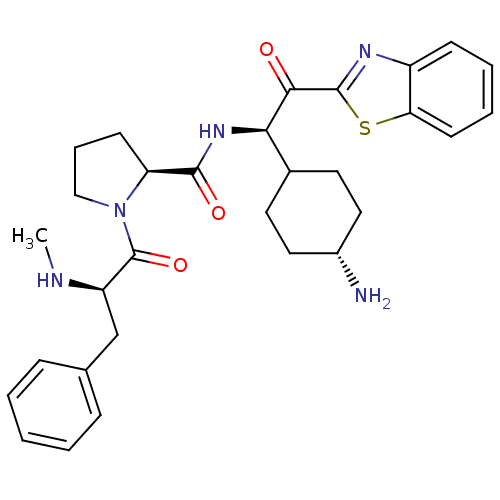
Inhibitor
Name:
BDBM14131
Synonyms:
(2S)-N-[(1R)-1-(4-aminocyclohexyl)-2-(1,3-benzothiazol-2-yl)-2-oxoethyl]-1-[(2R)-2-(methylamino)-3-phenylpropanoyl]pyrrolidine-2-carboxamide | 2-ketobenzothiazole 69 | The relative stereochemistry of the cyclohexyl ring is trans
Type:
Small organic molecule
Emp. Form.:
C30H37N5O3S
Mol. Mass.:
547.711
SMILES:
CN[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@H](C1CC[C@H](N)CC1)C(=O)c1nc2ccccc2s1 |r,wU:16.18,2.1,20.21,wD:24.26,(13.45,-1.07,;14.78,-.3,;16.11,-1.07,;16.11,-2.61,;14.78,-3.38,;13.45,-2.61,;12.11,-3.38,;12.11,-4.92,;13.45,-5.69,;14.78,-4.92,;17.45,-.3,;17.45,1.24,;18.78,-1.07,;18.78,.47,;20.12,1.24,;21.09,.27,;20.32,-1.07,;21.09,-2.4,;20.32,-3.73,;22.63,-2.4,;23.97,-3.17,;23.97,-4.71,;25.3,-5.48,;25.3,-7.02,;23.97,-7.79,;23.97,-9.9,;22.63,-7.02,;22.63,-5.48,;25.3,-2.4,;25.3,-.86,;26.63,-3.17,;27.88,-2.26,;29.12,-3.17,;30.63,-2.85,;31.66,-3.99,;31.18,-5.46,;29.68,-5.78,;28.65,-4.63,;27.11,-4.63,)|
Substrate
Name:
BDBM14064
Synonyms:
(2S)-2-[(2S)-2-[(2R)-2-amino-3-(4-hydroxycyclohexyl)propanamido]propanamido]-5-carbamimidamido-N-(4-nitrophenyl)pentanamide; bis(acetic acid) | H-D-HHT-Ala-Arg-pNA.2AcOH | Spectozyme TH
Type:
Small organic molecule
Emp. Form.:
C24H38N8O6
Mol. Mass.:
534.6085
SMILES:
CC(NC(=O)C(N)CC1CCC(O)CC1)C(=O)NC(CCC\[NH+]=C(\N)[NH-])C(=O)Nc1ccc(cc1)[N+]([O-])=O |(-2.35,2.49,;-1.58,3.83,;-2.35,5.16,;-3.89,5.16,;-4.66,6.49,;-4.66,3.83,;-6.2,3.83,;-3.88,2.49,;-4.65,1.16,;-6.2,1.16,;-6.97,-.17,;-6.2,-1.51,;-6.97,-2.84,;-4.65,-1.51,;-3.88,-.17,;-.04,3.83,;.73,5.16,;.73,2.49,;2.27,2.49,;3.04,1.16,;2.27,-.17,;3.04,-1.51,;2.27,-2.84,;3.04,-4.18,;2.27,-5.51,;4.58,-4.18,;3.04,3.83,;2.27,5.16,;4.58,3.83,;5.35,5.16,;4.58,6.49,;5.35,7.83,;6.9,7.83,;7.67,6.49,;6.9,5.16,;7.67,9.16,;9.21,9.16,;6.9,10.49,)|