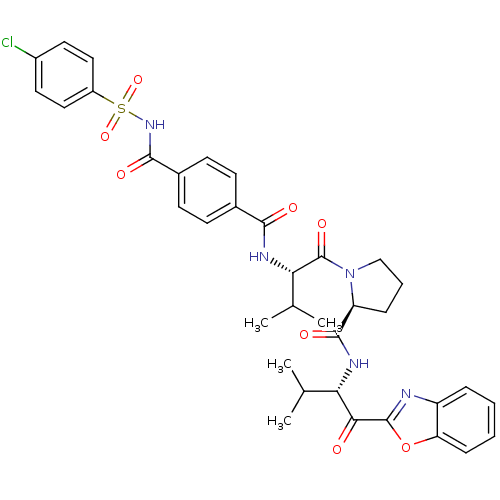
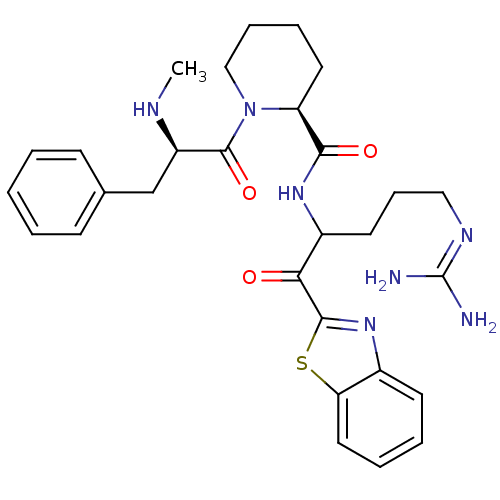
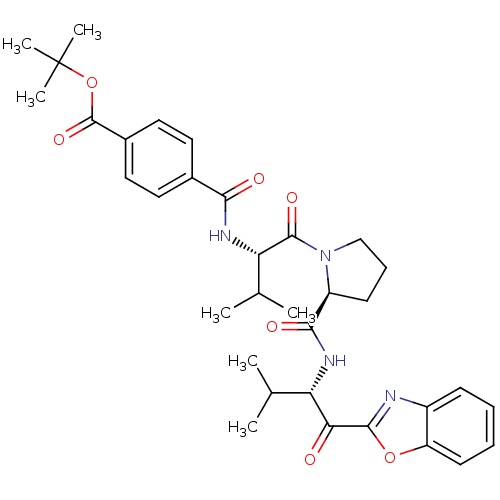
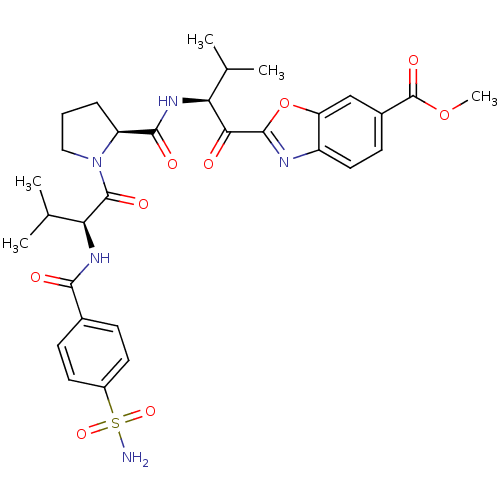
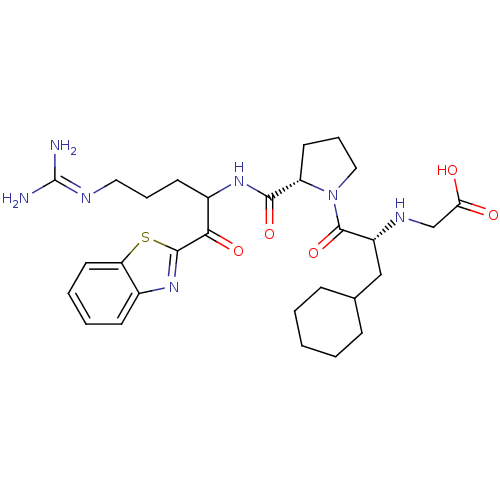
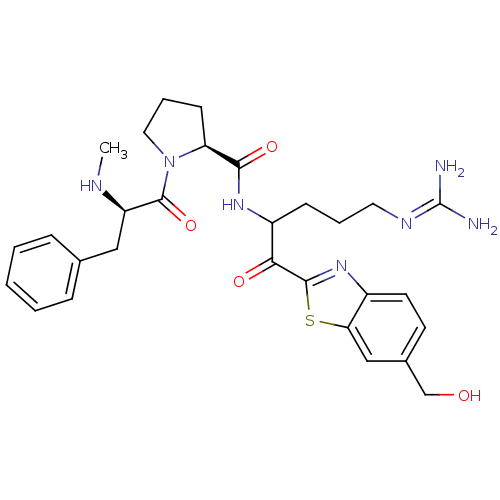
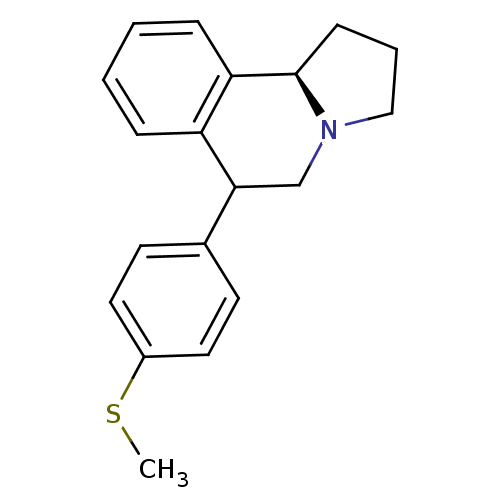
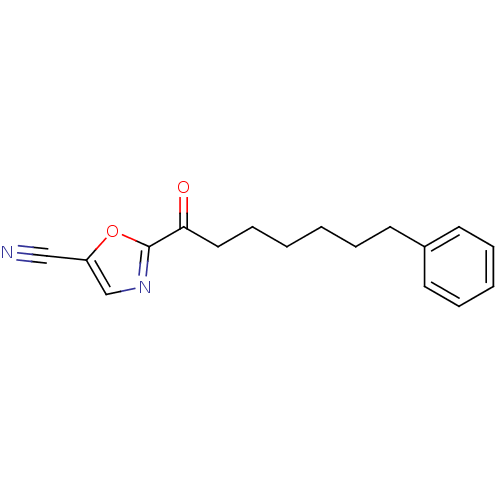
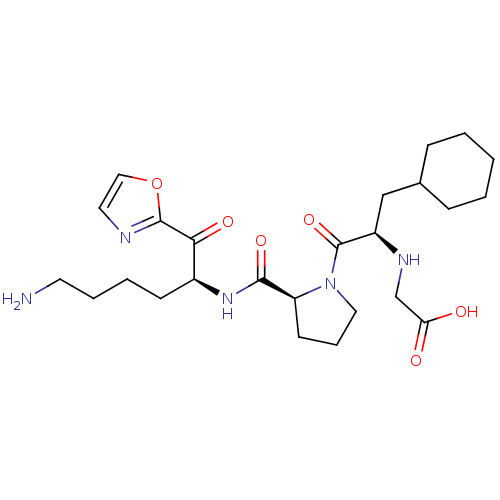
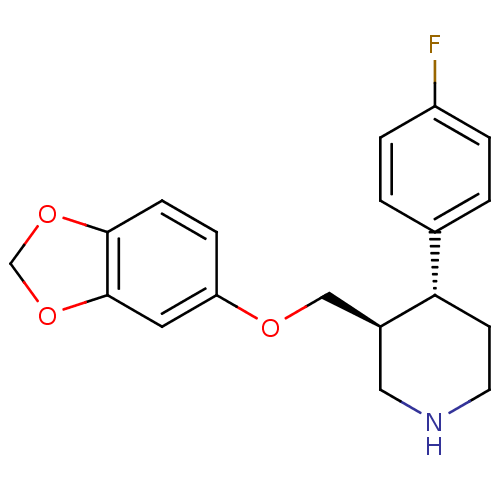
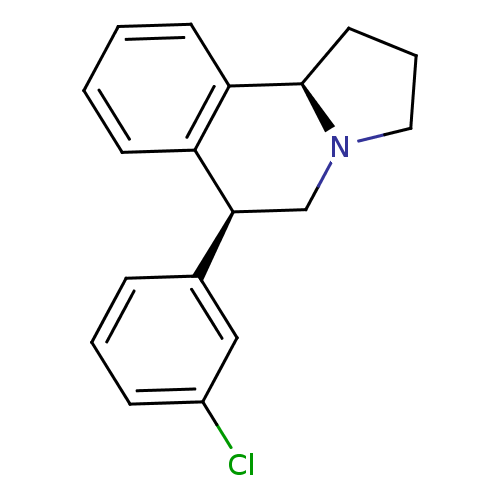
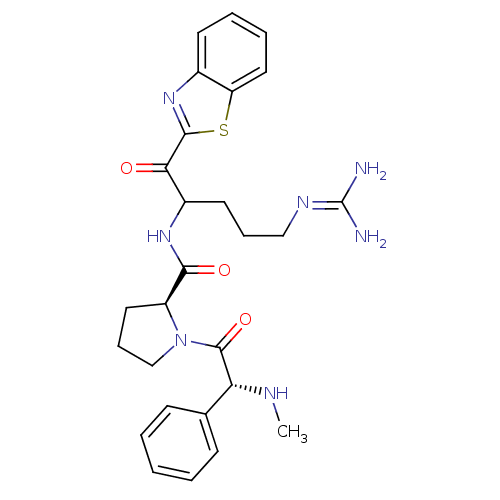
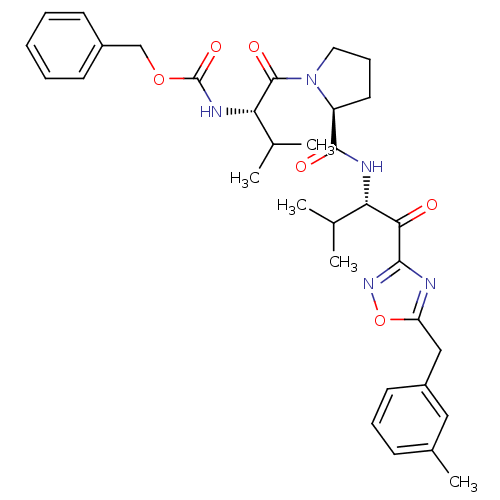
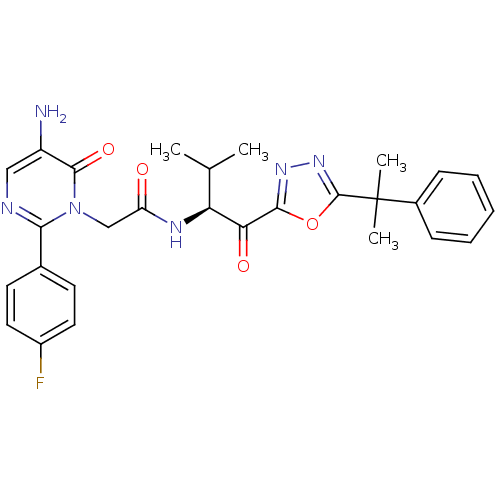
Affinity DataKi: 0.000650nM ΔG°: -72.4kJ/mole IC50: 4.5nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.000650nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.00550nM ΔG°: -66.9kJ/mole IC50: 21nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.00700nM IC50: 5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0140nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
Affinity DataKi: 0.0180nM ΔG°: -63.8kJ/mole IC50: 5.30nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0250nMAssay Description:Inhibition of human neutrophil elastaseMore data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0600nMAssay Description:Inhibition of human neutrophil elastaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.0710nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.0940nMAssay Description:Inhibition of human recombinant FAAHMore data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Inhibition of human neutrophil elastaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.120nM ΔG°: -58.9kJ/mole IC50: 15nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nM IC50: 5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:Binding affinity towards Dopamine receptor D1More data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.150nM IC50: 7.5nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.150nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Inhibition of uptake of tritiated norepinephrine (NE) in rat synaptosomesMore data for this Ligand-Target Pair
Affinity DataKi: 0.180nM ΔG°: -57.9kJ/mole IC50: 48nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.190nMAssay Description:Inhibition of human alpha-thrombin.More data for this Ligand-Target Pair
Affinity DataKi: 0.200nM ΔG°: -57.6kJ/mole IC50: 3.5nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nM ΔG°: -57.6kJ/mole IC50: 29nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Inhibition of human neutrophil elastaseMore data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Inhibition of rat FAAHMore data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Inhibition of human neutrophil elastaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Inhibition of rat FAAHMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Inhibition of uptake of tritiated norepinephrine (NE) in rat synaptosomesMore data for this Ligand-Target Pair
Affinity DataKi: 0.230nM IC50: 3nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.260nMAssay Description:Inhibition of human recombinant FAAHMore data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.290nMAssay Description:Inhibition of rat FAAHMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Trypsin-catalyzed hydrolysis rates were measured spectrophotometrically using bovine trypsin, a chromogenic substrate in aqueous buffer, and a microp...More data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.330nMAssay Description:Inhibition of human neutrophil elastaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.340nM IC50: 38nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.340nMAssay Description:Inhibition of human neutrophil elastaseMore data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.350nMAssay Description:Inhibition of human neutrophil elastaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.360nM ΔG°: -56.1kJ/mole IC50: 29nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.370nM IC50: 7nMAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
Affinity DataKi: 0.370nMAssay Description:Inhibition of uptake of tritiated norepinephrine (NE) in rat synaptosomesMore data for this Ligand-Target Pair
Affinity DataKi: 0.370nMAssay Description:Inhibition of uptake of tritiated norepinephrine (NE) in rat synaptosomesMore data for this Ligand-Target Pair
Affinity DataKi: 0.390nMAssay Description:Tested in vitro for serotonin(5-HT) neuronal uptake inhibitionMore data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1 [30-579](Rattus norvegicus (rat))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Inhibition of rat FAAHMore data for this Ligand-Target Pair
Affinity DataKi: 0.420nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.440nMAssay Description:Tested in vitro for dopamine(DA) neuronal uptake inhibitionMore data for this Ligand-Target Pair
Affinity DataKi: 0.450nMAssay Description:Inhibition of uptake of tritiated norepinephrine (NE) in rat synaptosomesMore data for this Ligand-Target Pair
Affinity DataKi: 0.460nM ΔG°: -55.4kJ/mole IC50: 34nMpH: 7.4 T: 2°CAssay Description:Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a...More data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.490nMAssay Description:Inhibition of human neutrophil elastaseMore data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.520nMAssay Description:Inhibition of human neutrophil elastaseMore data for this Ligand-Target Pair

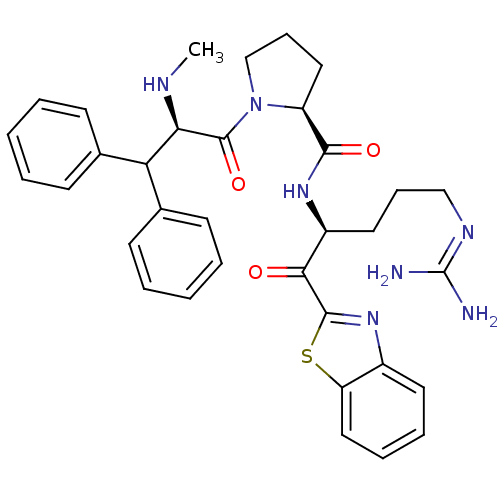
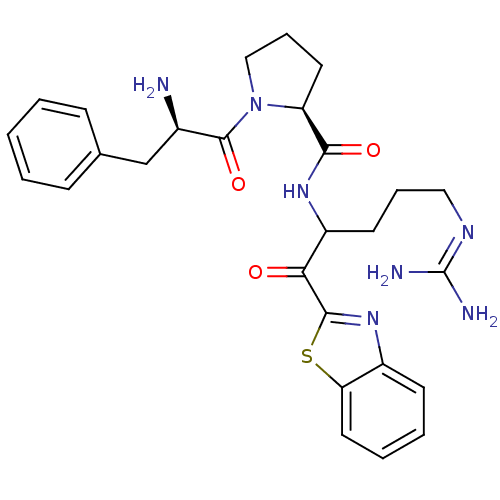
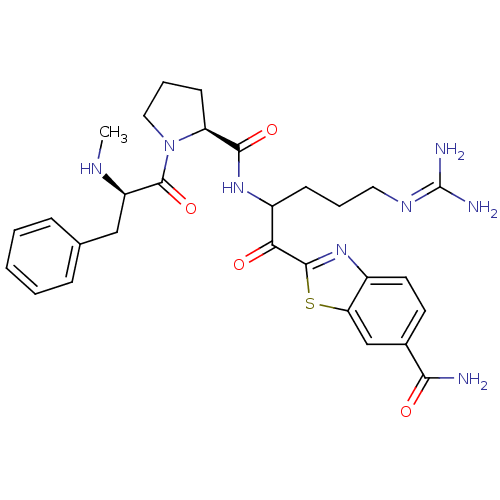
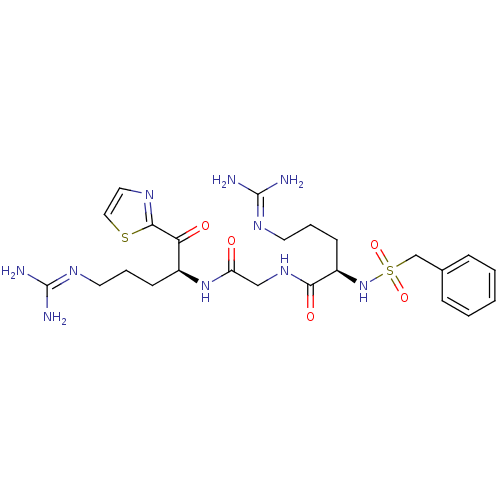
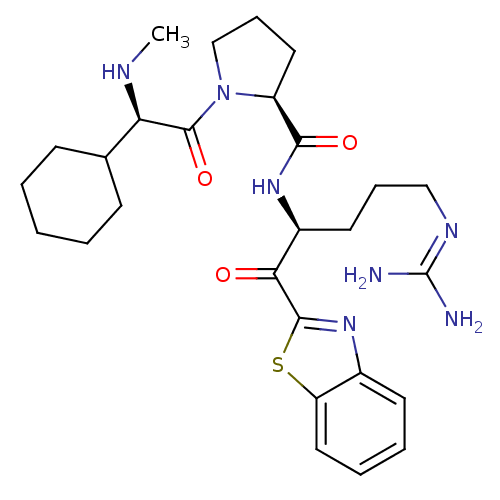
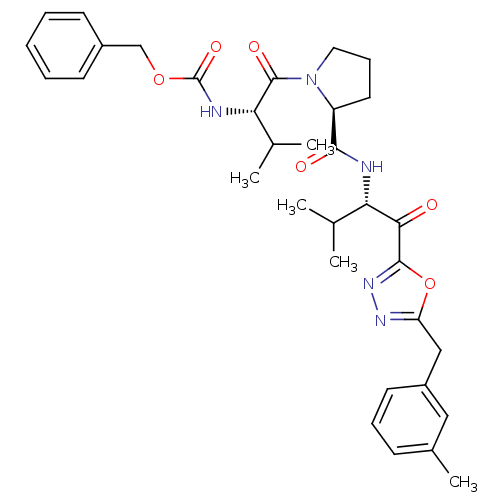
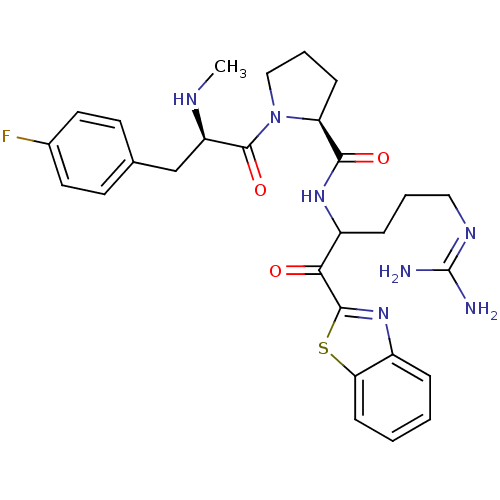
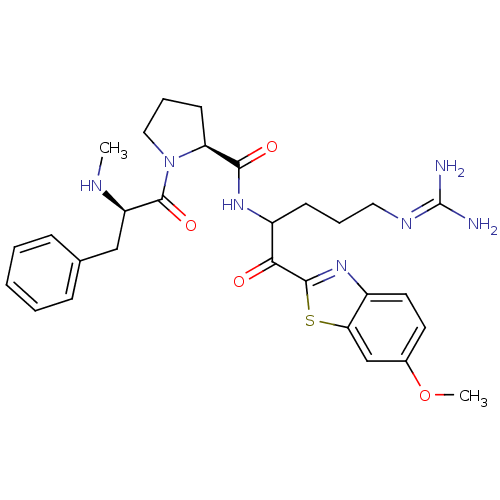
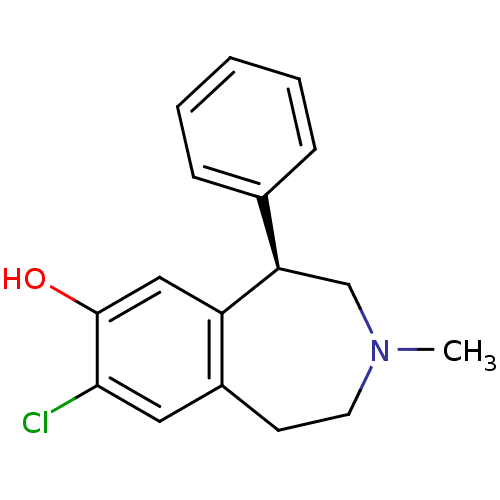
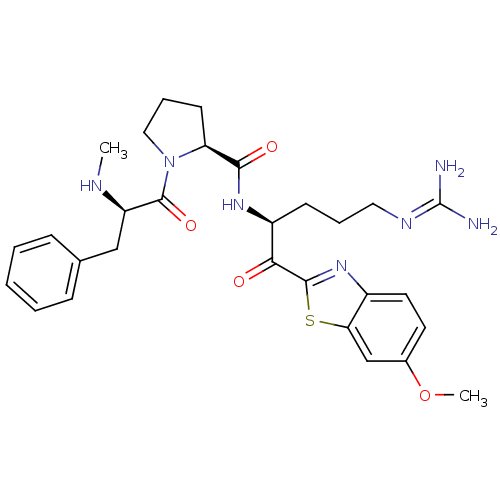
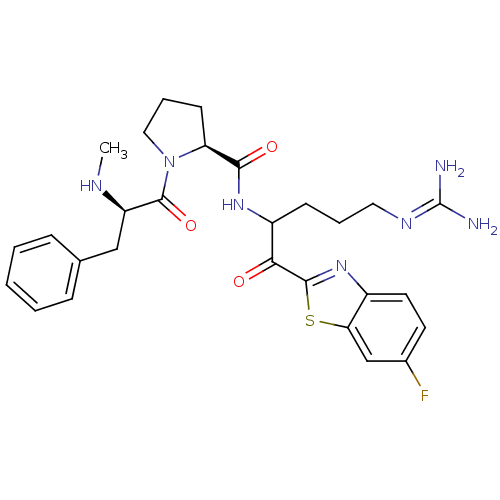
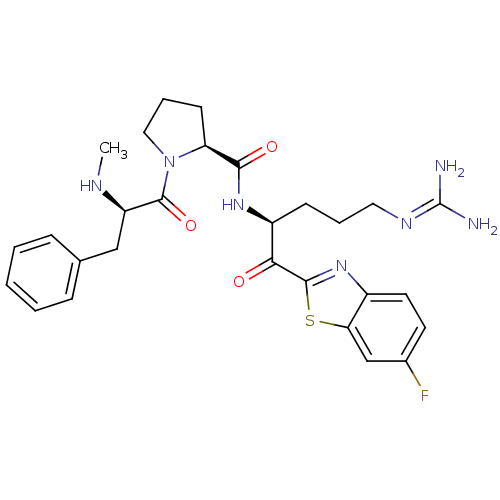
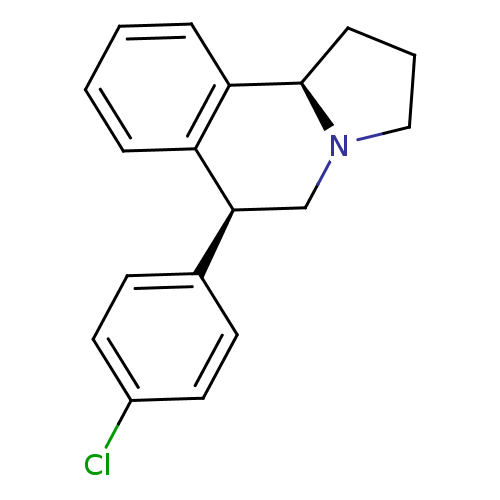
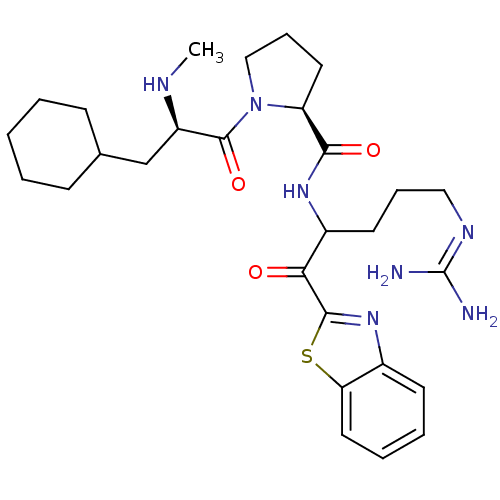
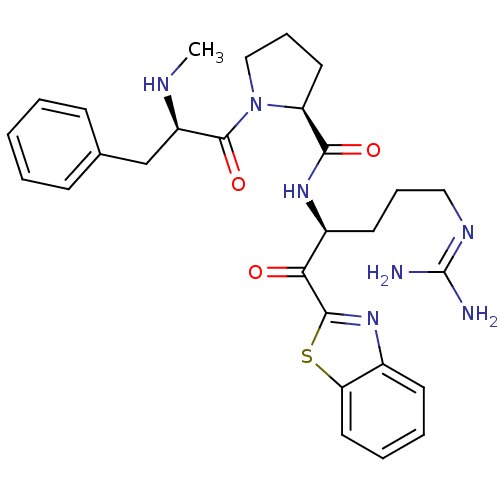
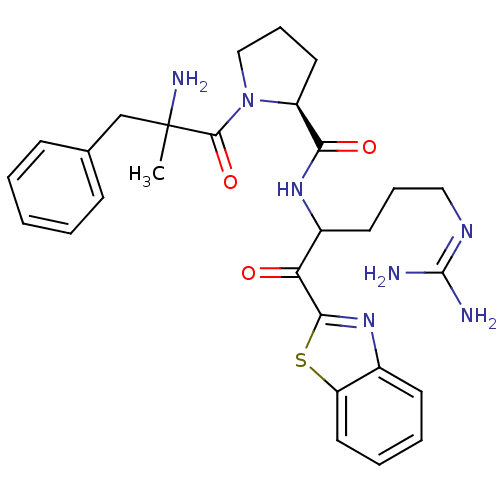
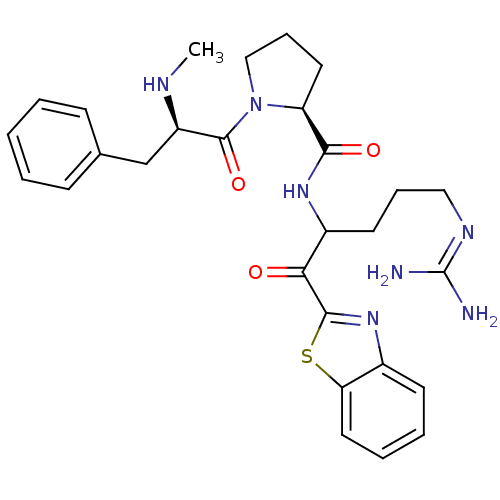
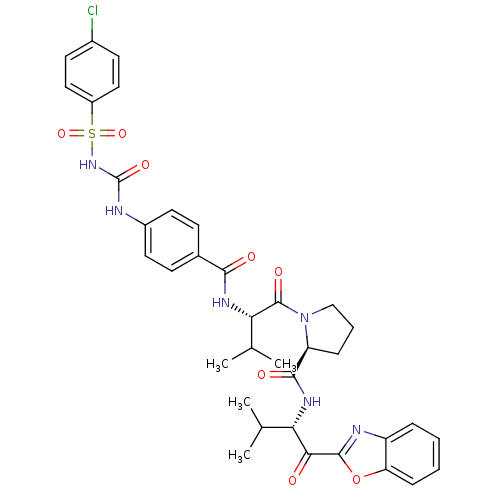
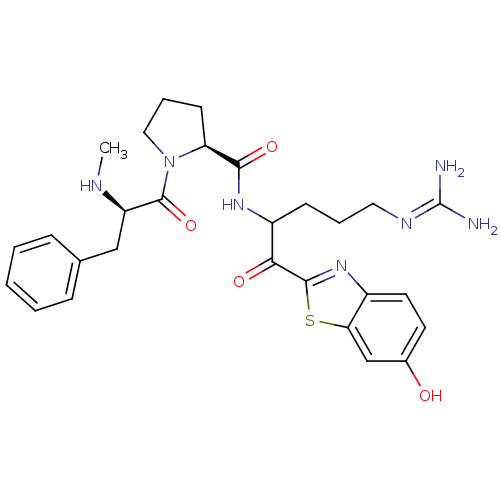
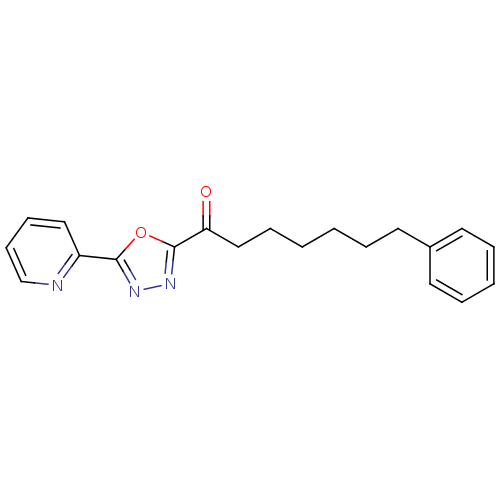
 3D Structure (crystal)
3D Structure (crystal)