Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 11B2, mitochondrial
Ligand
BDBM25442
Substrate
BDBM8582
Meas. Tech.
CYP11B1 Inhibition Assay
IC50
840±n/a nM
Citation
 Pinto-Bazurco Mendieta, MA; Negri, M; Jagusch, C; Müller-Vieira, U; Lauterbach, T; Hartmann, RW Synthesis, biological evaluation, and molecular modeling of abiraterone analogues: novel CYP17 inhibitors for the treatment of prostate cancer. J Med Chem 51:5009-18 (2008) [PubMed] Article
Pinto-Bazurco Mendieta, MA; Negri, M; Jagusch, C; Müller-Vieira, U; Lauterbach, T; Hartmann, RW Synthesis, biological evaluation, and molecular modeling of abiraterone analogues: novel CYP17 inhibitors for the treatment of prostate cancer. J Med Chem 51:5009-18 (2008) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 11B2, mitochondrial
Synonyms:
Aldosterone Synthase (CYP11B2) | Aldosterone synthase | Aldosterone-synthesizing enzyme | C11B2_HUMAN | CYP11B2 | CYPXIB2 | Cytochrome P450 11B2 | Cytochrome P450 11B2 (CYP11B2) | Cytochrome P450 11B2, mitochondrial | P-450Aldo | P-450C18 | Steroid 18-hydroxylase
Type:
Protein
Mol. Mass.:
57582.15
Organism:
Homo sapiens (Human)
Description:
P19099
Residue:
503
Sequence:
MALRAKAEVCVAAPWLSLQRARALGTRAARAPRTVLPFEAMPQHPGNRWLRLLQIWREQGYEHLHLEMHQTFQELGPIFRYNLGGPRMVCVMLPEDVEKLQQVDSLHPCRMILEPWVAYRQHRGHKCGVFLLNGPEWRFNRLRLNPDVLSPKAVQRFLPMVDAVARDFSQALKKKVLQNARGSLTLDVQPSIFHYTIEASNLALFGERLGLVGHSPSSASLNFLHALEVMFKSTVQLMFMPRSLSRWISPKVWKEHFEAWDCIFQYGDNCIQKIYQELAFNRPQHYTGIVAELLLKAELSLEAIKANSMELTAGSVDTTAFPLLMTLFELARNPDVQQILRQESLAAAASISEHPQKATTELPLLRAALKETLRLYPVGLFLERVVSSDLVLQNYHIPAGTLVQVFLYSLGRNAALFPRPERYNPQRWLDIRGSGRNFHHVPFGFGMRQCLGRRLAEAEMLLLLHHVLKHFLVETLTQEDIKMVYSFILRPGTSPLLTFRAIN
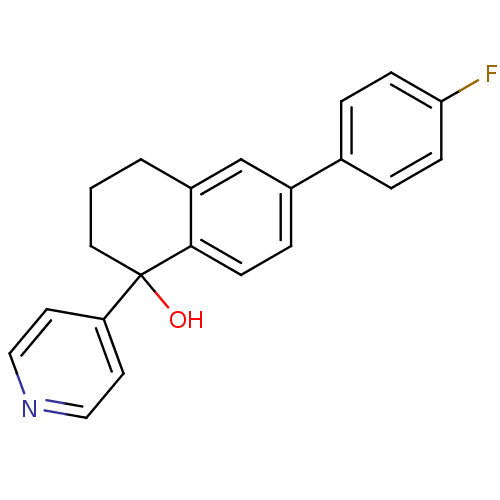
Inhibitor
Name:
BDBM25442
Synonyms:
6-(4-fluorophenyl)-1-(pyridin-4-yl)-1,2,3,4-tetrahydronaphthalen-1-ol | Abiraterone mimetic, 5
Type:
Small organic molecule
Emp. Form.:
C21H18FNO
Mol. Mass.:
319.3721
SMILES:
OC1(CCCc2cc(ccc12)-c1ccc(F)cc1)c1ccncc1
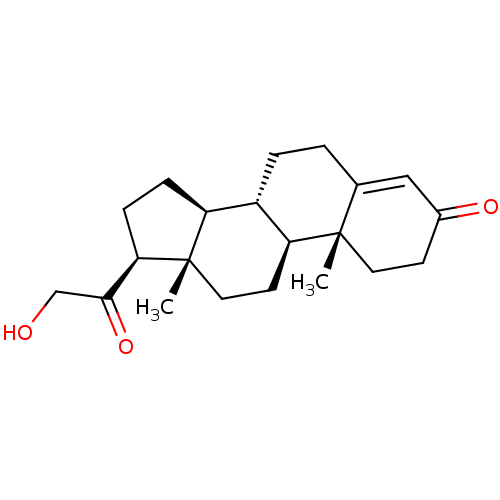
Substrate
Name:
BDBM8582
Synonyms:
(1S,2R,10S,11S,14S,15S)-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one | 11-deoxycorticosterone | 21-hydroxypregn-4-ene-3,20-dione | DOC | [4-14C]-11-deoxycorticosterone | deoxycorticosterone
Type:
Small organic molecule
Emp. Form.:
C21H30O3
Mol. Mass.:
330.4611
SMILES:
[H][C@@]12CC[C@H](C(=O)CO)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:21|