Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM8610
Substrate
BDBM8885
Meas. Tech.
CYP17 Inhibition Assay
IC50
72±n/a nM
Citation
 Pinto-Bazurco Mendieta, MA; Negri, M; Jagusch, C; Müller-Vieira, U; Lauterbach, T; Hartmann, RW Synthesis, biological evaluation, and molecular modeling of abiraterone analogues: novel CYP17 inhibitors for the treatment of prostate cancer. J Med Chem 51:5009-18 (2008) [PubMed] Article
Pinto-Bazurco Mendieta, MA; Negri, M; Jagusch, C; Müller-Vieira, U; Lauterbach, T; Hartmann, RW Synthesis, biological evaluation, and molecular modeling of abiraterone analogues: novel CYP17 inhibitors for the treatment of prostate cancer. J Med Chem 51:5009-18 (2008) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
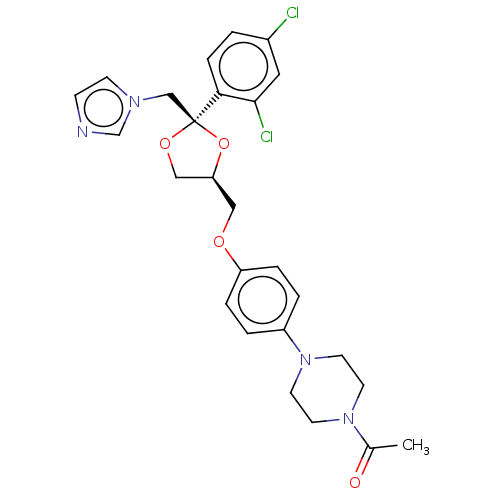
Inhibitor
Name:
BDBM8610
Synonyms:
1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy}phenyl)piperazin-1-yl]ethan-1-one | 24F2-1,25(OH)D3 | CHEMBL75 | KTZ | Ketoconazole | Ketoconazole (k) | US9138393, Ketoconozole | US9144538, Ketoconozole
Type:
Small organic molecule
Emp. Form.:
C26H28Cl2N4O4
Mol. Mass.:
531.431
SMILES:
[H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r|
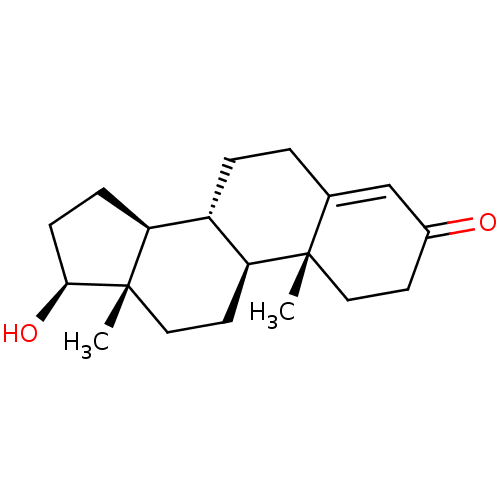
Substrate
Name:
BDBM8885
Synonyms:
(1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one | 17beta-Hydroxyandrost-4-en-3-one | Testosterone | Testosterone, 1 | US9682960, Testosterone
Type:
Steroid
Emp. Form.:
C19H28O2
Mol. Mass.:
288.4244
SMILES:
[H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:18|