Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
cGMP-dependent 3',5'-cyclic phosphodiesterase
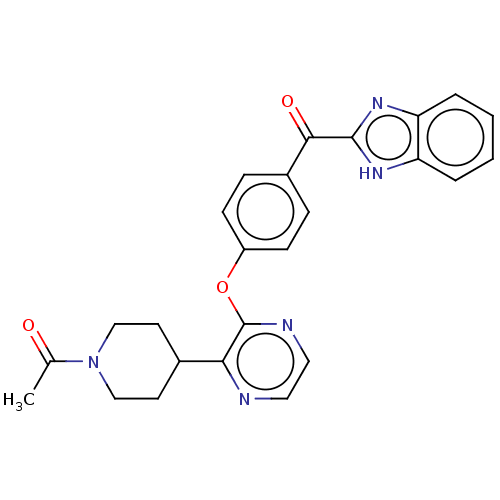
Ligand
BDBM50497974
Substrate
n/a
Meas. Tech.
ChEMBL_1446987 (CHEMBL3373744)
IC50
>30000±n/a nM
Citation
 Hu, E; Chen, N; Bourbeau, MP; Harrington, PE; Biswas, K; Kunz, RK; Andrews, KL; Chmait, S; Zhao, X; Davis, C; Ma, J; Shi, J; Lester-Zeiner, D; Danao, J; Able, J; Cueva, M; Talreja, S; Kornecook, T; Chen, H; Porter, A; Hungate, R; Treanor, J; Allen, JR Discovery of clinical candidate 1-(4-(3-(4-(1H-benzo[d]imidazole-2-carbonyl)phenoxy)pyrazin-2-yl)piperidin-1-yl)ethanone (AMG 579), a potent, selective, and efficacious inhibitor of phosphodiesterase 10A (PDE10A). J Med Chem 57:6632-41 (2014) [PubMed] Article
Hu, E; Chen, N; Bourbeau, MP; Harrington, PE; Biswas, K; Kunz, RK; Andrews, KL; Chmait, S; Zhao, X; Davis, C; Ma, J; Shi, J; Lester-Zeiner, D; Danao, J; Able, J; Cueva, M; Talreja, S; Kornecook, T; Chen, H; Porter, A; Hungate, R; Treanor, J; Allen, JR Discovery of clinical candidate 1-(4-(3-(4-(1H-benzo[d]imidazole-2-carbonyl)phenoxy)pyrazin-2-yl)piperidin-1-yl)ethanone (AMG 579), a potent, selective, and efficacious inhibitor of phosphodiesterase 10A (PDE10A). J Med Chem 57:6632-41 (2014) [PubMed] Article More Info.:
Target
Name:
cGMP-dependent 3',5'-cyclic phosphodiesterase
Synonyms:
CGS-PDE | Cyclic GMP-stimulated phosphodiesterase | Homo sapiens phosphodiesterase 2A (PDE2A) | NM_002599 | PDE2A | PDE2A_HUMAN | cGSPDE
Type:
Enzyme Catalytic Domain
Mol. Mass.:
105691.58
Organism:
Homo sapiens (Human)
Description:
O00408
Residue:
941
Sequence:
MGQACGHSILCRSQQYPAARPAEPRGQQVFLKPDEPPPPPQPCADSLQDALLSLGSVIDISGLQRAVKEALSAVLPRVETVYTYLLDGESQLVCEDPPHELPQEGKVREAIISQKRLGCNGLGFSDLPGKPLARLVAPLAPDTQVLVMPLADKEAGAVAAVILVHCGQLSDNEEWSLQAVEKHTLVALRRVQVLQQRGPREAPRAVQNPPEGTAEDQKGGAAYTDRDRKILQLCGELYDLDASSLQLKVLQYLQQETRASRCCLLLVSEDNLQLSCKVIGDKVLGEEVSFPLTGCLGQVVEDKKSIQLKDLTSEDVQQLQSMLGCELQAMLCVPVISRATDQVVALACAFNKLEGDLFTDEDEHVIQHCFHYTSTVLTSTLAFQKEQKLKCECQALLQVAKNLFTHLDDVSVLLQEIITEARNLSNAEICSVFLLDQNELVAKVFDGGVVDDESYEIRIPADQGIAGHVATTGQILNIPDAYAHPLFYRGVDDSTGFRTRNILCFPIKNENQEVIGVAELVNKINGPWFSKFDEDLATAFSIYCGISIAHSLLYKKVNEAQYRSHLANEMMMYHMKVSDDEYTKLLHDGIQPVAAIDSNFASFTYTPRSLPEDDTSMAILSMLQDMNFINNYKIDCPTLARFCLMVKKGYRDPPYHNWMHAFSVSHFCYLLYKNLELTNYLEDIEIFALFISCMCHDLDHRGTNNSFQVASKSVLAALYSSEGSVMERHHFAQAIAILNTHGCNIFDHFSRKDYQRMLDLMRDIILATDLAHHLRIFKDLQKMAEVGYDRNNKQHHRLLLCLLMTSCDLSDQTKGWKTTRKIAELIYKEFFSQGDLEKAMGNRPMEMMDREKAYIPELQISFMEHIAMPIYKLLQDLFPKAAELYERVASNREHWTKVSHKFTIRGLPSNNSLDFLDEEYEVPDLDGTRAPINGCCSLDAE