Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 1B1
Ligand
BDBM50503769
Substrate
n/a
Meas. Tech.
ChEMBL_1812125 (CHEMBL4311585)
IC50
68±n/a nM
Citation
 Kubo, M; Yamamoto, K; Itoh, T Design and synthesis of selective CYP1B1 inhibitor via dearomatization of ?-naphthoflavone. Bioorg Med Chem 27:285-304 (2019) [PubMed] Article
Kubo, M; Yamamoto, K; Itoh, T Design and synthesis of selective CYP1B1 inhibitor via dearomatization of ?-naphthoflavone. Bioorg Med Chem 27:285-304 (2019) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 1B1
Synonyms:
CP1B1_HUMAN | CYP1B1 | CYPIB1 | Cytochrome P450 1B1 (CYP1B1)
Type:
PROTEIN
Mol. Mass.:
60861.81
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1474523
Residue:
543
Sequence:
MGTSLSPNDPWPLNPLSIQQTTLLLLLSVLATVHVGQRLLRQRRRQLRSAPPGPFAWPLIGNAAAVGQAAHLSFARLARRYGDVFQIRLGSCPIVVLNGERAIHQALVQQGSAFADRPAFASFRVVSGGRSMAFGHYSEHWKVQRRAAHSMMRNFFTRQPRSRQVLEGHVLSEARELVALLVRGSADGAFLDPRPLTVVAVANVMSAVCFGCRYSHDDPEFRELLSHNEEFGRTVGAGSLVDVMPWLQYFPNPVRTVFREFEQLNRNFSNFILDKFLRHCESLRPGAAPRDMMDAFILSAEKKAAGDSHGGGARLDLENVPATITDIFGASQDTLSTALQWLLLLFTRYPDVQTRVQAELDQVVGRDRLPCMGDQPNLPYVLAFLYEAMRFSSFVPVTIPHATTANTSVLGYHIPKDTVVFVNQWSVNHDPLKWPNPENFDPARFLDKDGLINKDLTSRVMIFSVGKRRCIGEELSKMQLFLFISILAHQCDFRANPNEPAKMNFSYGLTIKPKSFKVNVTLRESMELLDSAVQNLQAKETCQ
Inhibitor
Name:
BDBM50503769
Synonyms:
CHEMBL4541853
Type:
Small organic molecule
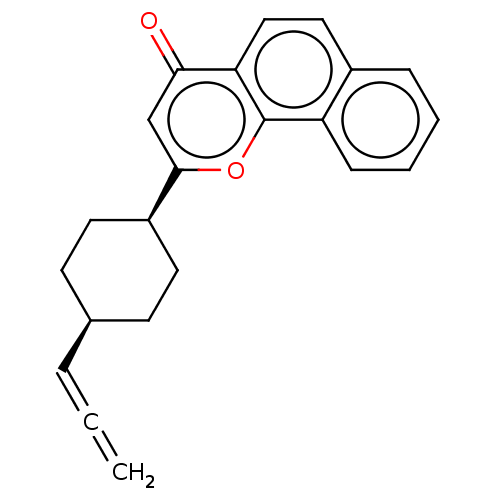
Emp. Form.:
C22H20O2
Mol. Mass.:
316.393
SMILES:
C=C=C[C@H]1CC[C@H](CC1)c1cc(=O)c2ccc3ccccc3c2o1 |r,wU:6.9,3.2,(58.87,-5.43,;57.55,-4.66,;56.22,-3.88,;54.88,-4.64,;54.87,-6.18,;53.53,-6.93,;52.21,-6.16,;52.21,-4.62,;53.55,-3.86,;50.88,-6.92,;50.87,-8.47,;49.53,-9.24,;49.53,-10.78,;48.2,-8.46,;46.87,-9.24,;45.53,-8.47,;45.53,-6.92,;44.21,-6.17,;44.2,-4.64,;45.53,-3.86,;46.86,-4.62,;46.86,-6.15,;48.2,-6.92,;49.53,-6.14,)|