Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Peroxisome proliferator-activated receptor gamma
Ligand
BDBM50075315
Substrate
n/a
Meas. Tech.
ChEMBL_220901 (CHEMBL824656)
EC50
1090±n/a nM
Citation
 Brooks, DA; Etgen, GJ; Rito, CJ; Shuker, AJ; Dominianni, SJ; Warshawsky, AM; Ardecky, R; Paterniti, JR; Tyhonas, J; Karanewsky, DS; Kauffman, RF; Broderick, CL; Oldham, BA; Montrose-Rafizadeh, C; Winneroski, LL; Faul, MM; McCarthy, JR Design and synthesis of 2-methyl-2-[4-(2-[5-methyl-2-aryloxazol-4-yl]ethoxy)phenoxy]propionic acids: a new class of dual PPARalpha/gamma agonists. J Med Chem 44:2061-4 (2001) [PubMed] Article
Brooks, DA; Etgen, GJ; Rito, CJ; Shuker, AJ; Dominianni, SJ; Warshawsky, AM; Ardecky, R; Paterniti, JR; Tyhonas, J; Karanewsky, DS; Kauffman, RF; Broderick, CL; Oldham, BA; Montrose-Rafizadeh, C; Winneroski, LL; Faul, MM; McCarthy, JR Design and synthesis of 2-methyl-2-[4-(2-[5-methyl-2-aryloxazol-4-yl]ethoxy)phenoxy]propionic acids: a new class of dual PPARalpha/gamma agonists. J Med Chem 44:2061-4 (2001) [PubMed] Article More Info.:
Target
Name:
Peroxisome proliferator-activated receptor gamma
Synonyms:
NR1C3 | Nuclear receptor subfamily 1 group C member 3 | PPAR-gamma | PPARG | PPARG_HUMAN | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor gamma (PPAR gamma) | Peroxisome proliferator-activated receptor gamma (PPARG) | Peroxisome proliferator-activated receptor gamma (PPARγ) | Peroxisome proliferator-activated receptor gamma/Nuclear receptor corepressor 2 | peroxisome proliferator-activated receptor gamma isoform 2
Type:
Nuclear Receptor
Mol. Mass.:
57613.46
Organism:
Homo sapiens (Human)
Description:
P37231
Residue:
505
Sequence:
MGETLGDSPIDPESDSFTDTLSANISQEMTMVDTEMPFWPTNFGISSVDLSVMEDHSHSFDIKPFTTVDFSSISTPHYEDIPFTRTDPVVADYKYDLKLQEYQSAIKVEPASPPYYSEKTQLYNKPHEEPSNSLMAIECRVCGDKASGFHYGVHACEGCKGFFRRTIRLKLIYDRCDLNCRIHKKSRNKCQYCRFQKCLAVGMSHNAIRFGRMPQAEKEKLLAEISSDIDQLNPESADLRALAKHLYDSYIKSFPLTKAKARAILTGKTTDKSPFVIYDMNSLMMGEDKIKFKHITPLQEQSKEVAIRIFQGCQFRSVEAVQEITEYAKSIPGFVNLDLNDQVTLLKYGVHEIIYTMLASLMNKDGVLISEGQGFMTREFLKSLRKPFGDFMEPKFEFAVKFNALELDDSDLAIFIAVIILSGDRPGLLNVKPIEDIQDNLLQALELQLKLNHPESSQLFAKLLQKMTDLRQIVTEHVQLLQVIKKTETDMSLHPLLQEIYKDLY
Inhibitor
Name:
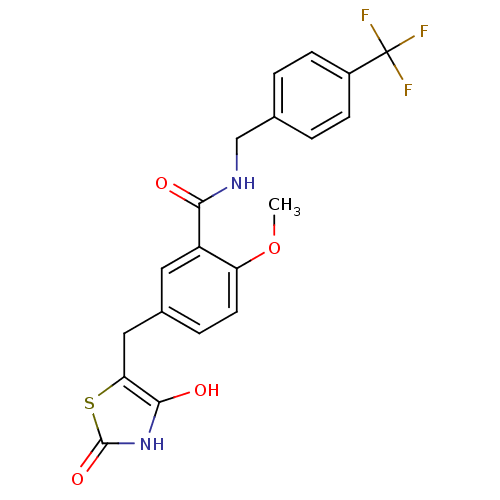
BDBM50075315
Synonyms:
5-(2,4-Dioxo-thiazolidin-5-ylmethyl)-2-methoxy-N-(4-trifluoromethyl-benzyl)-benzamide | 5-(2,4-Dioxo-thiazolidin-5-ylmethyl)-2-methoxy-N-(4-trifluoromethyl-benzyl)-benzamide(KRP297) | CHEMBL24458 | KRP-297
Type:
Small organic molecule
Emp. Form.:
C20H17F3N2O4S
Mol. Mass.:
438.42
SMILES:
COc1ccc(Cc2sc(=O)[nH]c2O)cc1C(=O)NCc1ccc(cc1)C(F)(F)F