Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Genome polyprotein
Ligand
BDBM50139678
Substrate
n/a
Meas. Tech.
ChEBML_79055
IC50
24000±n/a nM
Citation
 Chan, L; Pereira, O; Reddy, TJ; Das, SK; Poisson, C; Courchesne, M; Proulx, M; Siddiqui, A; Yannopoulos, CG; Nguyen-Ba, N; Roy, C; Nasturica, D; Moinet, C; Bethell, R; Hamel, M; L'Heureux, L; David, M; Nicolas, O; Courtemanche-Asselin, P; Brunette, S; Bilimoria, D; Bédard, J Discovery of thiophene-2-carboxylic acids as potent inhibitors of HCV NS5B polymerase and HCV subgenomic RNA replication. Part 2: tertiary amides. Bioorg Med Chem Lett 14:797-800 (2004) [PubMed] Article
Chan, L; Pereira, O; Reddy, TJ; Das, SK; Poisson, C; Courchesne, M; Proulx, M; Siddiqui, A; Yannopoulos, CG; Nguyen-Ba, N; Roy, C; Nasturica, D; Moinet, C; Bethell, R; Hamel, M; L'Heureux, L; David, M; Nicolas, O; Courtemanche-Asselin, P; Brunette, S; Bilimoria, D; Bédard, J Discovery of thiophene-2-carboxylic acids as potent inhibitors of HCV NS5B polymerase and HCV subgenomic RNA replication. Part 2: tertiary amides. Bioorg Med Chem Lett 14:797-800 (2004) [PubMed] Article More Info.:
Target
Name:
Genome polyprotein
Synonyms:
Genome polyprotein | Genome polyprotein (NS3-NS4A) | Hepatitis C virus polyprotein | POLG_HCV1 | RNA-dependent RNA polymerase (NS5B)
Type:
Protein
Mol. Mass.:
327266.82
Organism:
Hepatitis C virus (HCV)
Description:
P26664
Residue:
3011
Sequence:
MSTNPKPQKKNKRNTNRRPQDVKFPGGGQIVGGVYLLPRRGPRLGVRATRKTSERSQPRGRRQPIPKARRPEGRTWAQPGYPWPLYGNEGCGWAGWLLSPRGSRPSWGPTDPRRRSRNLGKVIDTLTCGFADLMGYIPLVGAPLGGAARALAHGVRVLEDGVNYATGNLPGCSFSIFLLALLSCLTVPASAYQVRNSTGLYHVTNDCPNSSIVYEAADAILHTPGCVPCVREGNASRCWVAMTPTVATRDGKLPATQLRRHIDLLVGSATLCSALYVGDLCGSVFLVGQLFTFSPRRHWTTQGCNCSIYPGHITGHRMAWDMMMNWSPTTALVMAQLLRIPQAILDMIAGAHWGVLAGIAYFSMVGNWAKVLVVLLLFAGVDAETHVTGGSAGHTVSGFVSLLAPGAKQNVQLINTNGSWHLNSTALNCNDSLNTGWLAGLFYHHKFNSSGCPERLASCRPLTDFDQGWGPISYANGSGPDQRPYCWHYPPKPCGIVPAKSVCGPVYCFTPSPVVVGTTDRSGAPTYSWGENDTDVFVLNNTRPPLGNWFGCTWMNSTGFTKVCGAPPCVIGGAGNNTLHCPTDCFRKHPDATYSRCGSGPWITPRCLVDYPYRLWHYPCTINYTIFKIRMYVGGVEHRLEAACNWTRGERCDLEDRDRSELSPLLLTTTQWQVLPCSFTTLPALSTGLIHLHQNIVDVQYLYGVGSSIASWAIKWEYVVLLFLLLADARVCSCLWMMLLISQAEAALENLVILNAASLAGTHGLVSFLVFFCFAWYLKGKWVPGAVYTFYGMWPLLLLLLALPQRAYALDTEVAASCGGVVLVGLMALTLSPYYKRYISWCLWWLQYFLTRVEAQLHVWIPPLNVRGGRDAVILLMCAVHPTLVFDITKLLLAVFGPLWILQASLLKVPYFVRVQGLLRFCALARKMIGGHYVQMVIIKLGALTGTYVYNHLTPLRDWAHNGLRDLAVAVEPVVFSQMETKLITWGADTAACGDIINGLPVSARRGREILLGPADGMVSKGWRLLAPITAYAQQTRGLLGCIITSLTGRDKNQVEGEVQIVSTAAQTFLATCINGVCWTVYHGAGTRTIASPKGPVIQMYTNVDQDLVGWPAPQGSRSLTPCTCGSSDLYLVTRHADVIPVRRRGDSRGSLLSPRPISYLKGSSGGPLLCPAGHAVGIFRAAVCTRGVAKAVDFIPVENLETTMRSPVFTDNSSPPVVPQSFQVAHLHAPTGSGKSTKVPAAYAAQGYKVLVLNPSVAATLGFGAYMSKAHGIDPNIRTGVRTITTGSPITYSTYGKFLADGGCSGGAYDIIICDECHSTDATSILGIGTVLDQAETAGARLVVLATATPPGSVTVPHPNIEEVALSTTGEIPFYGKAIPLEVIKGGRHLIFCHSKKKCDELAAKLVALGINAVAYYRGLDVSVIPTSGDVVVVATDALMTGYTGDFDSVIDCNTCVTQTVDFSLDPTFTIETITLPQDAVSRTQRRGRTGRGKPGIYRFVAPGERPSGMFDSSVLCECYDAGCAWYELTPAETTVRLRAYMNTPGLPVCQDHLEFWEGVFTGLTHIDAHFLSQTKQSGENLPYLVAYQATVCARAQAPPPSWDQMWKCLIRLKPTLHGPTPLLYRLGAVQNEITLTHPVTKYIMTCMSADLEVVTSTWVLVGGVLAALAAYCLSTGCVVIVGRVVLSGKPAIIPDREVLYREFDEMEECSQHLPYIEQGMMLAEQFKQKALGLLQTASRQAEVIAPAVQTNWQKLETFWAKHMWNFISGIQYLAGLSTLPGNPAIASLMAFTAAVTSPLTTSQTLLFNILGGWVAAQLAAPGAATAFVGAGLAGAAIGSVGLGKVLIDILAGYGAGVAGALVAFKIMSGEVPSTEDLVNLLPAILSPGALVVGVVCAAILRRHVGPGEGAVQWMNRLIAFASRGNHVSPTHYVPESDAAARVTAILSSLTVTQLLRRLHQWISSECTTPCSGSWLRDIWDWICEVLSDFKTWLKAKLMPQLPGIPFVSCQRGYKGVWRVDGIMHTRCHCGAEITGHVKNGTMRIVGPRTCRNMWSGTFPINAYTTGPCTPLPAPNYTFALWRVSAEEYVEIRQVGDFHYVTGMTTDNLKCPCQVPSPEFFTELDGVRLHRFAPPCKPLLREEVSFRVGLHEYPVGSQLPCEPEPDVAVLTSMLTDPSHITAEAAGRRLARGSPPSVASSSASQLSAPSLKATCTANHDSPDAELIEANLLWRQEMGGNITRVESENKVVILDSFDPLVAEEDEREISVPAEILRKSRRFAQALPVWARPDYNPPLVETWKKPDYEPPVVHGCPLPPPKSPPVPPPRKKRTVVLTESTLSTALAELATRSFGSSSTSGITGDNTTTSSEPAPSGCPPDSDAESYSSMPPLEGEPGDPDLSDGSWSTVSSEANAEDVVCCSMSYSWTGALVTPCAAEEQKLPINALSNSLLRHHNLVYSTTSRSACQRQKKVTFDRLQVLDSHYQDVLKEVKAAASKVKANLLSVEEACSLTPPHSAKSKFGYGAKDVRCHARKAVTHINSVWKDLLEDNVTPIDTTIMAKNEVFCVQPEKGGRKPARLIVFPDLGVRVCEKMALYDVVTKLPLAVMGSSYGFQYSPGQRVEFLVQAWKSKKTPMGFSYDTRCFDSTVTESDIRTEEAIYQCCDLDPQARVAIKSLTERLYVGGPLTNSRGENCGYRRCRASGVLTTSCGNTLTCYIKARAACRAAGLQDCTMLVCGDDLVVICESAGVQEDAASLRAFTEAMTRYSAPPGDPPQPEYDLELITSCSSNVSVAHDGAGKRVYYLTRDPTTPLARAAWETARHTPVNSWLGNIIMFAPTLWARMILMTHFFSVLIARDQLEQALDCEIYGACYSIEPLDLPPIIQRLHGLSAFSLHSYSPGEINRVAACLRKLGVPPLRAWRHRARSVRARLLARGGRAAICGKYLFNWAVRTKLKLTPIAAAGQLDLSGWFTAGYSGGDIYHSVSHARPRWIWFCLLLLAAGVGIYLLPNR
Inhibitor
Name:
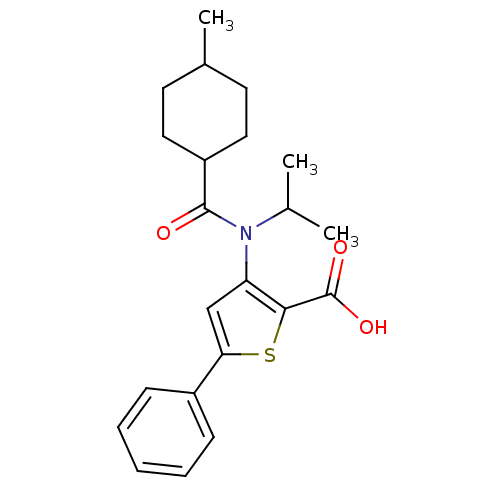
BDBM50139678
Synonyms:
3-[Isopropyl-(4-methyl-cyclohexanecarbonyl)-amino]-5-phenyl-thiophene-2-carboxylic acid | 3-{ISOPROPYL[(TRANS-4-METHYLCYCLOHEXYL)CARBONYL]AMINO}-5-PHENYLTHIOPHENE-2-CARBOXYLIC ACID | CHEMBL352911
Type:
Small organic molecule
Emp. Form.:
C22H27NO3S
Mol. Mass.:
385.52
SMILES:
CC(C)N(C(=O)C1CCC(C)CC1)c1cc(sc1C(O)=O)-c1ccccc1 |(6.22,3.55,;5.82,2.07,;7.32,1.67,;4.33,1.66,;3.25,2.75,;3.64,4.24,;1.76,2.35,;.5,1.93,;-1.14,2.55,;-1.91,1.19,;-1.91,-.35,;-.72,1.61,;1,1,;3.95,.16,;2.52,-.4,;2.6,-1.92,;4.1,-2.32,;4.94,-1.04,;6.47,-.94,;7.32,-2.23,;7.16,.44,;1.41,-2.9,;1.67,-4.42,;.48,-5.4,;-.97,-4.84,;-1.21,-3.32,;-.02,-2.36,)|