Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Tyrosine-protein kinase BTK
Ligand
BDBM291522
Substrate
n/a
Meas. Tech.
ChEMBL_2158481 (CHEMBL5043231)
Kd
4.6±n/a nM
Citation
 Hopkins, BT; Bame, E; Bajrami, B; Black, C; Bohnert, T; Boiselle, C; Burdette, D; Burns, JC; Delva, L; Donaldson, D; Grater, R; Gu, C; Hoemberger, M; Johnson, J; Kapadnis, S; King, K; Lulla, M; Ma, B; Marx, I; Magee, T; Meissner, R; Metrick, CM; Mingueneau, M; Murugan, P; Otipoby, KL; Polack, E; Poreci, U; Prince, R; Roach, AM; Rowbottom, C; Santoro, JC; Schroeder, P; Tang, H; Tien, E; Zhang, F; Lyssikatos, J Discovery and Preclinical Characterization of BIIB091, a Reversible, Selective BTK Inhibitor for the Treatment of Multiple Sclerosis. J Med Chem 65:1206-1224 (2022) [PubMed] Article
Hopkins, BT; Bame, E; Bajrami, B; Black, C; Bohnert, T; Boiselle, C; Burdette, D; Burns, JC; Delva, L; Donaldson, D; Grater, R; Gu, C; Hoemberger, M; Johnson, J; Kapadnis, S; King, K; Lulla, M; Ma, B; Marx, I; Magee, T; Meissner, R; Metrick, CM; Mingueneau, M; Murugan, P; Otipoby, KL; Polack, E; Poreci, U; Prince, R; Roach, AM; Rowbottom, C; Santoro, JC; Schroeder, P; Tang, H; Tien, E; Zhang, F; Lyssikatos, J Discovery and Preclinical Characterization of BIIB091, a Reversible, Selective BTK Inhibitor for the Treatment of Multiple Sclerosis. J Med Chem 65:1206-1224 (2022) [PubMed] Article More Info.:
Target
Name:
Tyrosine-protein kinase BTK
Synonyms:
AGMX1 | ATK | Agammaglobulinaemia tyrosine kinase | Agammaglobulinemia tyrosine kinase | B cell progenitor kinase | B-cell progenitor kinase | BPK | BTK | BTK_HUMAN | Bruton tyrosine kinase | Tyrosine Kinase BTK | Tyrosine-protein kinase (BTK) | Tyrosine-protein kinase BTK (BTK)
Type:
Enzyme
Mol. Mass.:
76289.95
Organism:
Homo sapiens (Human)
Description:
Q06187
Residue:
659
Sequence:
MAAVILESIFLKRSQQKKKTSPLNFKKRLFLLTVHKLSYYEYDFERGRRGSKKGSIDVEKITCVETVVPEKNPPPERQIPRRGEESSEMEQISIIERFPYPFQVVYDEGPLYVFSPTEELRKRWIHQLKNVIRYNSDLVQKYHPCFWIDGQYLCCSQTAKNAMGCQILENRNGSLKPGSSHRKTKKPLPPTPEEDQILKKPLPPEPAAAPVSTSELKKVVALYDYMPMNANDLQLRKGDEYFILEESNLPWWRARDKNGQEGYIPSNYVTEAEDSIEMYEWYSKHMTRSQAEQLLKQEGKEGGFIVRDSSKAGKYTVSVFAKSTGDPQGVIRHYVVCSTPQSQYYLAEKHLFSTIPELINYHQHNSAGLISRLKYPVSQQNKNAPSTAGLGYGSWEIDPKDLTFLKELGTGQFGVVKYGKWRGQYDVAIKMIKEGSMSEDEFIEEAKVMMNLSHEKLVQLYGVCTKQRPIFIITEYMANGCLLNYLREMRHRFQTQQLLEMCKDVCEAMEYLESKQFLHRDLAARNCLVNDQGVVKVSDFGLSRYVLDDEYTSSVGSKFPVRWSPPEVLMYSKFSSKSDIWAFGVLMWEIYSLGKMPYERFTNSETAEHIAQGLRLYRPHLASEKVYTIMYSCWHEKADERPTFKILLSNILDVMDEES
Inhibitor
Name:
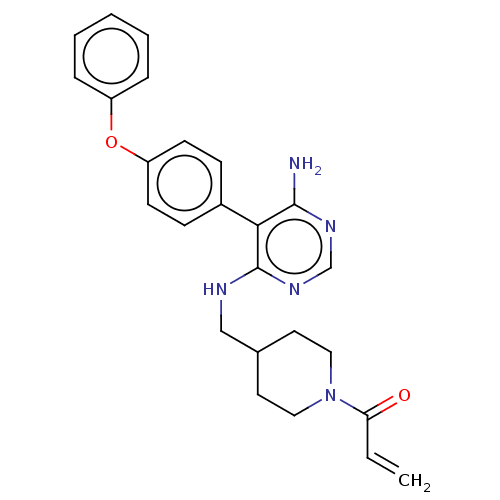
BDBM291522
Synonyms:
1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)amino)methyl)piperidin-1-yl)prop-2-en-1-one | US10413562, Compound A250 | US9580449, Example A250 | US9580449, Example A39
Type:
Small organic molecule
Emp. Form.:
C25H27N5O2
Mol. Mass.:
429.5142
SMILES:
Nc1ncnc(NCC2CCN(CC2)C(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1