Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
UDP-N-acetylenolpyruvoylglucosamine reductase
Ligand
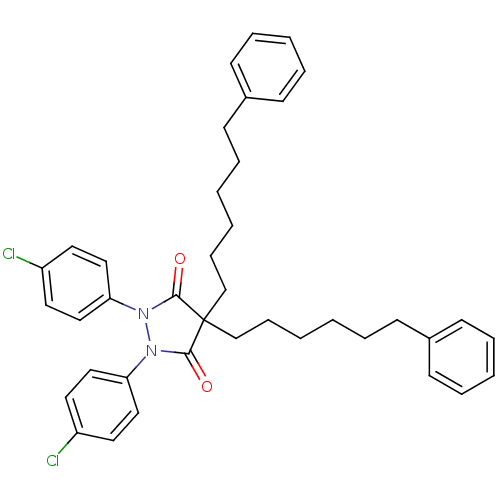
BDBM50166475
Substrate
n/a
Meas. Tech.
ChEMBL_304867 (CHEMBL829244)
IC50
8200.0±n/a nM
Citation
 Kutterer, KM; Davis, JM; Singh, G; Yang, Y; Hu, W; Severin, A; Rasmussen, BA; Krishnamurthy, G; Failli, A; Katz, AH 4-Alkyl and 4,4'-dialkyl 1,2-bis(4-chlorophenyl)pyrazolidine-3,5-dione derivatives as new inhibitors of bacterial cell wall biosynthesis. Bioorg Med Chem Lett 15:2527-31 (2005) [PubMed] Article
Kutterer, KM; Davis, JM; Singh, G; Yang, Y; Hu, W; Severin, A; Rasmussen, BA; Krishnamurthy, G; Failli, A; Katz, AH 4-Alkyl and 4,4'-dialkyl 1,2-bis(4-chlorophenyl)pyrazolidine-3,5-dione derivatives as new inhibitors of bacterial cell wall biosynthesis. Bioorg Med Chem Lett 15:2527-31 (2005) [PubMed] Article More Info.:
Target
Name:
UDP-N-acetylenolpyruvoylglucosamine reductase
Synonyms:
MURB_STAAU | MurB (S. aureus) | UDP-N-acetylenolpyruvoylglucosamine reductase | murB
Type:
Protein
Mol. Mass.:
33791.48
Organism:
Staphylococcus aureus (Firmicutes)
Description:
S. aureus MurB
Residue:
307
Sequence:
MINKDIYQALQQLIPNEKIKVDEPLKRYTYTKTGGNADFYITPTKNEEVQAVVKYAYQNEIPVTYLGNGSNIIIREGGIRGIVISLLSLDHIEVSDDAIIAGSGAAIIDVSRVARDYALTGLEFACGIPGSIGGAVYMNAGAYGGEVKDCIDYALCVNEQGSLIKLTTKELELDYRNSIIQKEHLVVLEAAFTLAPGKMTEIQAKMDDLTERRESKQPLEYPSCGSVFQRPPGHFAGKLIQDSNLQGHRIGGVEVSTKHAGFMVNVDNGTATDYENLIHYVQKTVKEKFGIELNREVRIIGEHPKES