Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Tyrosine-protein kinase BTK
Ligand
BDBM468007
Substrate
n/a
Meas. Tech.
ChEMBL_2238755 (CHEMBL5152651)
Ki
145±n/a nM
Citation
 Tichenor, MS; Wiener, JJM; Rao, NL; Bacani, GM; Wei, J; Pooley Deckhut, C; Barbay, JK; Kreutter, KD; Chang, L; Clancy, KW; Murrey, HE; Wang, W; Ahn, K; Huber, M; Rex, E; Coe, KJ; Wu, J; Rui, H; Sepassi, K; Gaudiano, M; Bekkers, M; Cornelissen, I; Packman, K; Seierstad, M; Xiouras, C; Bembenek, SD; Alexander, R; Milligan, C; Balasubramanian, S; Lebsack, AD; Venable, JD; Philippar, U; Edwards, JP; Hirst, G Discovery of JNJ-64264681: A Potent and Selective Covalent Inhibitor of Bruton's Tyrosine Kinase. J Med Chem 65:14326-14336 (2022) [PubMed]
Tichenor, MS; Wiener, JJM; Rao, NL; Bacani, GM; Wei, J; Pooley Deckhut, C; Barbay, JK; Kreutter, KD; Chang, L; Clancy, KW; Murrey, HE; Wang, W; Ahn, K; Huber, M; Rex, E; Coe, KJ; Wu, J; Rui, H; Sepassi, K; Gaudiano, M; Bekkers, M; Cornelissen, I; Packman, K; Seierstad, M; Xiouras, C; Bembenek, SD; Alexander, R; Milligan, C; Balasubramanian, S; Lebsack, AD; Venable, JD; Philippar, U; Edwards, JP; Hirst, G Discovery of JNJ-64264681: A Potent and Selective Covalent Inhibitor of Bruton's Tyrosine Kinase. J Med Chem 65:14326-14336 (2022) [PubMed] More Info.:
Target
Name:
Tyrosine-protein kinase BTK
Synonyms:
AGMX1 | ATK | Agammaglobulinaemia tyrosine kinase | Agammaglobulinemia tyrosine kinase | B cell progenitor kinase | B-cell progenitor kinase | BPK | BTK | BTK_HUMAN | Bruton tyrosine kinase | Tyrosine Kinase BTK | Tyrosine-protein kinase (BTK) | Tyrosine-protein kinase BTK (BTK)
Type:
Enzyme
Mol. Mass.:
76289.95
Organism:
Homo sapiens (Human)
Description:
Q06187
Residue:
659
Sequence:
MAAVILESIFLKRSQQKKKTSPLNFKKRLFLLTVHKLSYYEYDFERGRRGSKKGSIDVEKITCVETVVPEKNPPPERQIPRRGEESSEMEQISIIERFPYPFQVVYDEGPLYVFSPTEELRKRWIHQLKNVIRYNSDLVQKYHPCFWIDGQYLCCSQTAKNAMGCQILENRNGSLKPGSSHRKTKKPLPPTPEEDQILKKPLPPEPAAAPVSTSELKKVVALYDYMPMNANDLQLRKGDEYFILEESNLPWWRARDKNGQEGYIPSNYVTEAEDSIEMYEWYSKHMTRSQAEQLLKQEGKEGGFIVRDSSKAGKYTVSVFAKSTGDPQGVIRHYVVCSTPQSQYYLAEKHLFSTIPELINYHQHNSAGLISRLKYPVSQQNKNAPSTAGLGYGSWEIDPKDLTFLKELGTGQFGVVKYGKWRGQYDVAIKMIKEGSMSEDEFIEEAKVMMNLSHEKLVQLYGVCTKQRPIFIITEYMANGCLLNYLREMRHRFQTQQLLEMCKDVCEAMEYLESKQFLHRDLAARNCLVNDQGVVKVSDFGLSRYVLDDEYTSSVGSKFPVRWSPPEVLMYSKFSSKSDIWAFGVLMWEIYSLGKMPYERFTNSETAEHIAQGLRLYRPHLASEKVYTIMYSCWHEKADERPTFKILLSNILDVMDEES
Inhibitor
Name:
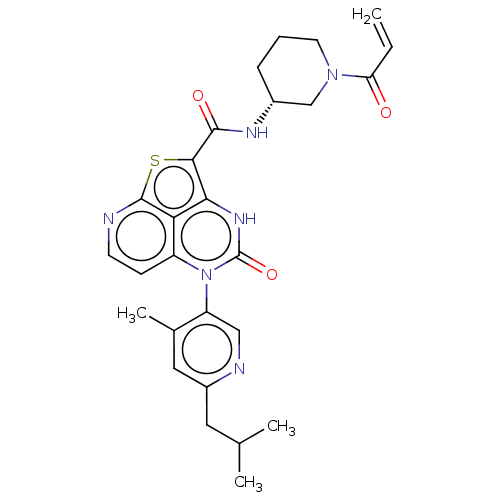
BDBM468007
Synonyms:
(R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(6-isobutyl-4- methylpyridin-3-yl)-4-oxo-4,5-dihydro-3H-1-thia-3,5,8- triazaacenaphthylene-2-carboxamide; | US10800792, Example 669 | US10822348, Example 722
Type:
Small organic molecule
Emp. Form.:
C27H30N6O3S
Mol. Mass.:
518.631
SMILES:
CC(C)Cc1cc(C)c(cn1)-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:22.22,(-9.16,1.88,;-9.16,3.42,;-10.5,4.19,;-7.83,4.19,;-6.5,3.42,;-6.5,1.88,;-5.16,1.11,;-5.16,-.49,;-3.83,1.88,;-3.83,3.42,;-5.16,4.19,;-2.5,1.11,;-2.5,-.43,;-3.83,-1.2,;-3.83,-2.74,;-2.5,-3.51,;-1.16,-2.74,;.32,-3.14,;1.26,-1.52,;2.8,-1.52,;3.57,-2.85,;3.57,-.19,;5.11,-.19,;5.88,1.15,;7.42,1.15,;8.19,-.19,;7.42,-1.52,;5.88,-1.52,;8.19,-2.85,;7.42,-4.19,;9.73,-2.85,;10.5,-1.52,;.17,-.43,;.17,1.11,;-1.16,1.88,;-1.16,3.42,;-1.16,-1.2,)|