Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Tyrosine-protein kinase BTK
Ligand
BDBM468084
Substrate
n/a
Meas. Tech.
ChEMBL_2238755 (CHEMBL5152651)
Ki
2550±n/a nM
Citation
 Tichenor, MS; Wiener, JJM; Rao, NL; Bacani, GM; Wei, J; Pooley Deckhut, C; Barbay, JK; Kreutter, KD; Chang, L; Clancy, KW; Murrey, HE; Wang, W; Ahn, K; Huber, M; Rex, E; Coe, KJ; Wu, J; Rui, H; Sepassi, K; Gaudiano, M; Bekkers, M; Cornelissen, I; Packman, K; Seierstad, M; Xiouras, C; Bembenek, SD; Alexander, R; Milligan, C; Balasubramanian, S; Lebsack, AD; Venable, JD; Philippar, U; Edwards, JP; Hirst, G Discovery of JNJ-64264681: A Potent and Selective Covalent Inhibitor of Bruton's Tyrosine Kinase. J Med Chem 65:14326-14336 (2022) [PubMed]
Tichenor, MS; Wiener, JJM; Rao, NL; Bacani, GM; Wei, J; Pooley Deckhut, C; Barbay, JK; Kreutter, KD; Chang, L; Clancy, KW; Murrey, HE; Wang, W; Ahn, K; Huber, M; Rex, E; Coe, KJ; Wu, J; Rui, H; Sepassi, K; Gaudiano, M; Bekkers, M; Cornelissen, I; Packman, K; Seierstad, M; Xiouras, C; Bembenek, SD; Alexander, R; Milligan, C; Balasubramanian, S; Lebsack, AD; Venable, JD; Philippar, U; Edwards, JP; Hirst, G Discovery of JNJ-64264681: A Potent and Selective Covalent Inhibitor of Bruton's Tyrosine Kinase. J Med Chem 65:14326-14336 (2022) [PubMed] More Info.:
Target
Name:
Tyrosine-protein kinase BTK
Synonyms:
AGMX1 | ATK | Agammaglobulinaemia tyrosine kinase | Agammaglobulinemia tyrosine kinase | B cell progenitor kinase | B-cell progenitor kinase | BPK | BTK | BTK_HUMAN | Bruton tyrosine kinase | Tyrosine Kinase BTK | Tyrosine-protein kinase (BTK) | Tyrosine-protein kinase BTK (BTK)
Type:
Enzyme
Mol. Mass.:
76289.95
Organism:
Homo sapiens (Human)
Description:
Q06187
Residue:
659
Sequence:
MAAVILESIFLKRSQQKKKTSPLNFKKRLFLLTVHKLSYYEYDFERGRRGSKKGSIDVEKITCVETVVPEKNPPPERQIPRRGEESSEMEQISIIERFPYPFQVVYDEGPLYVFSPTEELRKRWIHQLKNVIRYNSDLVQKYHPCFWIDGQYLCCSQTAKNAMGCQILENRNGSLKPGSSHRKTKKPLPPTPEEDQILKKPLPPEPAAAPVSTSELKKVVALYDYMPMNANDLQLRKGDEYFILEESNLPWWRARDKNGQEGYIPSNYVTEAEDSIEMYEWYSKHMTRSQAEQLLKQEGKEGGFIVRDSSKAGKYTVSVFAKSTGDPQGVIRHYVVCSTPQSQYYLAEKHLFSTIPELINYHQHNSAGLISRLKYPVSQQNKNAPSTAGLGYGSWEIDPKDLTFLKELGTGQFGVVKYGKWRGQYDVAIKMIKEGSMSEDEFIEEAKVMMNLSHEKLVQLYGVCTKQRPIFIITEYMANGCLLNYLREMRHRFQTQQLLEMCKDVCEAMEYLESKQFLHRDLAARNCLVNDQGVVKVSDFGLSRYVLDDEYTSSVGSKFPVRWSPPEVLMYSKFSSKSDIWAFGVLMWEIYSLGKMPYERFTNSETAEHIAQGLRLYRPHLASEKVYTIMYSCWHEKADERPTFKILLSNILDVMDEES
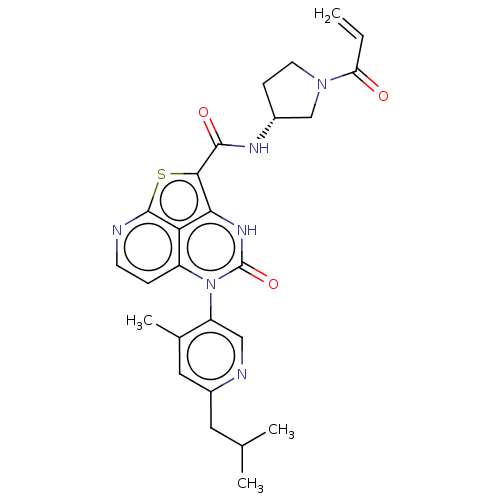
Inhibitor
Name:
BDBM468084
Synonyms:
(R)-N-(1-Acryloylpyrrolidin-3-yl)-5-(*S)-(6-isobutyl-4- methylpyridin-3-yl)-4-oxo-4,5-dihydro-3H-1-thia-3,5,8- triazaacenaphthylene-2-carboxamide; | US10800792, Example 747 | US10822348, Example 756
Type:
Small organic molecule
Emp. Form.:
C26H28N6O3S
Mol. Mass.:
504.604
SMILES:
CC(C)Cc1cc(C)c(cn1)-n1c2ccnc3sc(C(=O)N[C@@H]4CCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:22.22,(-10.96,3.85,;-9.63,3.08,;-9.63,1.54,;-8.3,3.85,;-6.96,3.08,;-6.96,1.54,;-5.63,.77,;-5.63,-.81,;-4.29,1.54,;-4.29,3.08,;-5.63,3.85,;-2.96,.77,;-2.96,-.77,;-4.29,-1.54,;-4.29,-3.08,;-2.96,-3.85,;-1.63,-3.08,;-.16,-3.56,;.74,-1.95,;2.28,-1.95,;3.05,-3.28,;3.05,-.61,;4.59,-.61,;5.5,.63,;6.96,.16,;6.96,-1.38,;5.5,-1.86,;8.3,-2.15,;8.3,-3.69,;9.63,-1.38,;10.96,-2.15,;-.29,-.77,;-.29,.77,;-1.63,1.54,;-1.63,3.08,;-1.63,-1.54,)|