Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Potassium voltage-gated channel subfamily H member 2
Ligand
BDBM50220723
Substrate
n/a
Meas. Tech.
ChEMBL_449357 (CHEMBL899624)
IC50
>30000±n/a nM
Citation
 Kawai, M; Nakamura, H; Sakurada, I; Shimokawa, H; Tanaka, H; Matsumizu, M; Ando, K; Hattori, K; Ohta, A; Nukui, S; Omura, A; Kawamura, M Discovery of novel and orally active NR2B-selective N-methyl-D-aspartate (NMDA) antagonists, pyridinol derivatives with reduced HERG binding affinity. Bioorg Med Chem Lett 17:5533-6 (2007) [PubMed] Article
Kawai, M; Nakamura, H; Sakurada, I; Shimokawa, H; Tanaka, H; Matsumizu, M; Ando, K; Hattori, K; Ohta, A; Nukui, S; Omura, A; Kawamura, M Discovery of novel and orally active NR2B-selective N-methyl-D-aspartate (NMDA) antagonists, pyridinol derivatives with reduced HERG binding affinity. Bioorg Med Chem Lett 17:5533-6 (2007) [PubMed] Article More Info.:
Target
Name:
Potassium voltage-gated channel subfamily H member 2
Synonyms:
1,3-beta-glucan synthase component GLS2 | Cytochrome P450 3A4 | ERG | ERG1 | Eag-related protein 1 | Ether a-go-go related gene potassium channel (hERG) | Ether-a-go-go-related gene (HERG) | Ether-a-go-go-related gene potassium channel (hERG) | Ether-a-go-go-related gene potassium channel 1 | Ether-a-go-go-related gene potassium channel 1 (HERG) | Ether-a-go-go-related gene potassium channel 1 (hERG1) | Ether-a-go-go-related protein (hERG) | Ether-a-go-go-related protein 1 | Ether-a-go-go-related protein 1 (HERG) | H-ERG | HERG | KCNH2 | KCNH2_HUMAN | Potassium voltage-gated channel subfamily H member 2 (hERG) | Transcriptional regulator ERG | Voltage-gated potassium channel subunit Kv11.1 | eag homolog | hERG Potassium Channel 1 | putative potassium channel subunit
Type:
Multi-pass membrane protein
Mol. Mass.:
126672.65
Organism:
Homo sapiens (Human)
Description:
Q12809
Residue:
1159
Sequence:
MPVRRGHVAPQNTFLDTIIRKFEGQSRKFIIANARVENCAVIYCNDGFCELCGYSRAEVMQRPCTCDFLHGPRTQRRAAAQIAQALLGAEERKVEIAFYRKDGSCFLCLVDVVPVKNEDGAVIMFILNFEVVMEKDMVGSPAHDTNHRGPPTSWLAPGRAKTFRLKLPALLALTARESSVRSGGAGGAGAPGAVVVDVDLTPAAPSSESLALDEVTAMDNHVAGLGPAEERRALVGPGSPPRSAPGQLPSPRAHSLNPDASGSSCSLARTRSRESCASVRRASSADDIEAMRAGVLPPPPRHASTGAMHPLRSGLLNSTSDSDLVRYRTISKIPQITLNFVDLKGDPFLASPTSDREIIAPKIKERTHNVTEKVTQVLSLGADVLPEYKLQAPRIHRWTILHYSPFKAVWDWLILLLVIYTAVFTPYSAAFLLKETEEGPPATECGYACQPLAVVDLIVDIMFIVDILINFRTTYVNANEEVVSHPGRIAVHYFKGWFLIDMVAAIPFDLLIFGSGSEELIGLLKTARLLRLVRVARKLDRYSEYGAAVLFLLMCTFALIAHWLACIWYAIGNMEQPHMDSRIGWLHNLGDQIGKPYNSSGLGGPSIKDKYVTALYFTFSSLTSVGFGNVSPNTNSEKIFSICVMLIGSLMYASIFGNVSAIIQRLYSGTARYHTQMLRVREFIRFHQIPNPLRQRLEEYFQHAWSYTNGIDMNAVLKGFPECLQADICLHLNRSLLQHCKPFRGATKGCLRALAMKFKTTHAPPGDTLVHAGDLLTALYFISRGSIEILRGDVVVAILGKNDIFGEPLNLYARPGKSNGDVRALTYCDLHKIHRDDLLEVLDMYPEFSDHFWSSLEITFNLRDTNMIPGSPGSTELEGGFSRQRKRKLSFRRRTDKDTEQPGEVSALGPGRAGAGPSSRGRPGGPWGESPSSGPSSPESSEDEGPGRSSSPLRLVPFSSPRPPGEPPGGEPLMEDCEKSSDTCNPLSGAFSGVSNIFSFWGDSRGRQYQELPRCPAPTPSLLNIPLSSPGRRPRGDVESRLDALQRQLNRLETRLSADMATVLQLLQRQMTLVPPAYSAVTTPGPGPTSTSPLLPVSPLPTLTLDSLSQVSQFMACEELPPGAPELPQEGPTRRLSLPGQLGALTSQPLHRHGSDPGS
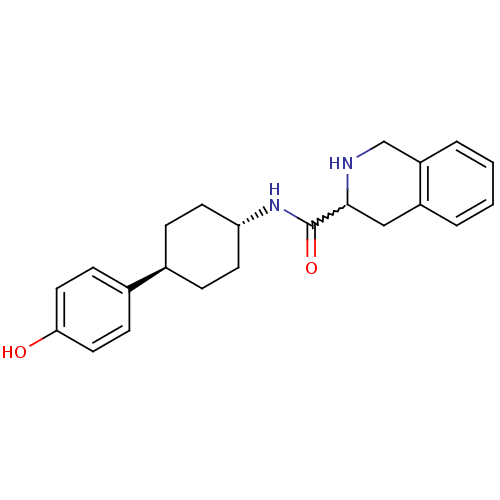
Inhibitor
Name:
BDBM50220723
Synonyms:
CHEMBL237102 | N-((1r,4r)-4-(4-hydroxyphenyl)cyclohexyl)-1,2,3,4-tetrahydroisoquinoline-3-carboxamide
Type:
Small organic molecule
Emp. Form.:
C22H26N2O2
Mol. Mass.:
350.454
SMILES:
Oc1ccc(cc1)[C@H]1CC[C@@H](CC1)NC(=O)C1Cc2ccccc2CN1 |w:16.17,wU:7.7,wD:10.14,(35.06,-11.3,;36.39,-10.54,;36.39,-8.98,;37.71,-8.22,;39.05,-8.99,;39.06,-10.52,;37.74,-11.3,;40.39,-8.21,;40.39,-6.67,;41.71,-5.88,;43.05,-6.67,;43.05,-8.21,;41.71,-8.98,;44.39,-5.9,;45.72,-6.67,;45.72,-8.22,;47.07,-5.91,;48.39,-6.69,;49.73,-5.93,;51.05,-6.71,;52.39,-5.96,;52.4,-4.41,;51.08,-3.64,;49.74,-4.39,;48.41,-3.61,;47.07,-4.37,)|