Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Neurotensin receptor type 1
Ligand
BDBM50130880
Substrate
n/a
Meas. Tech.
ChEMBL_562557 (CHEMBL1014549)
EC50
2±n/a nM
Citation
 Orwig, KS; Lassetter, MR; Hadden, MK; Dix, TA Comparison of N-terminal modifications on neurotensin(8-13) analogues correlates peptide stability but not binding affinity with in vivo efficacy. J Med Chem 52:1803-13 (2009) [PubMed] Article
Orwig, KS; Lassetter, MR; Hadden, MK; Dix, TA Comparison of N-terminal modifications on neurotensin(8-13) analogues correlates peptide stability but not binding affinity with in vivo efficacy. J Med Chem 52:1803-13 (2009) [PubMed] Article More Info.:
Target
Name:
Neurotensin receptor type 1
Synonyms:
NT-R-1 | NTR1_MOUSE | Neurotensin 1 | Neurotensin receptor 1 | Neurotensin receptor type 1 | Ntsr | Ntsr1
Type:
Enzyme Catalytic Domain
Mol. Mass.:
47237.04
Organism:
MOUSE
Description:
Neurotensin 1 NTSR1 MOUSE::A2ACT4
Residue:
424
Sequence:
MHLNSSVQQGAPSEPGAQPFPHPQFGLETMLLALSLSNGSGNSSESILEPNSNLDVNTDIYSKVLVTAVYLALFVVGTVGNSVTAFTLARKKSLQSLQSTVHYHLGSLALSDLLILLLAMPVELYNFIWVHHPWAFGDAGCRGYYFLRDACTYATALNVASLSVERYLAICHPFKAKTLMSRSRTKKFISAIWLASALLAVPMLFTMGLQNRSADGQHPGGLVCTPTVDTATVKVVIQVNTFMSFLFPMLIISILNTVIANKLTVMVHQAAEQGRGVCTVGTHNSLEHSTFNMSIEPGRVQALRHGVLVLRAVVIAFVVCWLPYHVRRLMFCYISDEQWTTFLFDFYHYFYMLTNALFYVSSAINPILYNLVSANFRQVFLSTLACLCPGWRRRRKKRPTFSRKPNSMSSNHAFSTSATRETLY
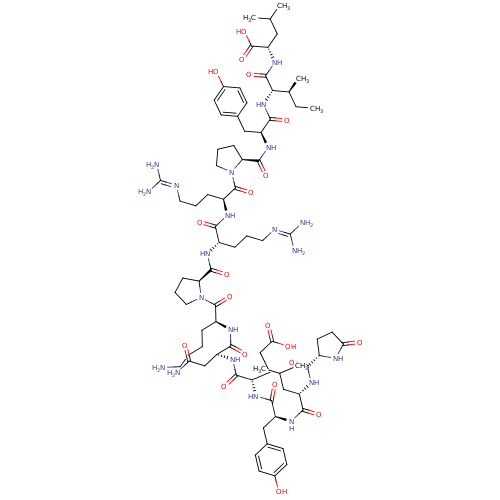
Inhibitor
Name:
BDBM50130880
Synonyms:
CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-Tyr-Glu-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu | pGlu-Leu-Tyr-Glu-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu-OH
Type:
Small organic molecule
Emp. Form.:
C78H121N21O20
Mol. Mass.:
1672.924
SMILES:
[#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r|