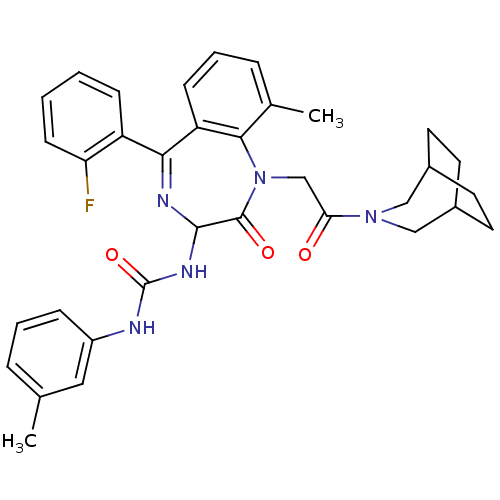
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cholecystokinin receptor type A
Ligand
BDBM50290396
Substrate
n/a
Meas. Tech.
ChEMBL_50046 (CHEMBL662415)
IC50
17±n/a nM
Citation
 Tabuchi, S; Ito, H; Sogabe, H; Kuno, M; Katsumi, I; Yamamoto, N; Mitsui, H; Satoh, Y Dual CCK-A and -B receptor antagonists (I) C9-methyl-1,4-benzodiazepines Bioorg Med Chem Lett 7:169-174 (1997) Article
Tabuchi, S; Ito, H; Sogabe, H; Kuno, M; Katsumi, I; Yamamoto, N; Mitsui, H; Satoh, Y Dual CCK-A and -B receptor antagonists (I) C9-methyl-1,4-benzodiazepines Bioorg Med Chem Lett 7:169-174 (1997) Article More Info.:
Target
Name:
Cholecystokinin receptor type A
Synonyms:
CCKAR_RAT | Cckar | Cholecystokinin peripheral | Cholecystokinin receptor | Cholecystokinin receptor type A
Type:
Enzyme Catalytic Domain
Mol. Mass.:
49676.37
Organism:
RAT
Description:
Cholecystokinin central 0 RAT::P30551
Residue:
444
Sequence:
MSHSPARQHLVESSRMDVVDSLLMNGSNITPPCELGLENETLFCLDQPQPSKEWQSALQILLYSIIFLLSVLGNTLVITVLIRNKRMRTVTNIFLLSLAVSDLMLCLFCMPFNLIPNLLKDFIFGSAVCKTTTYFMGTSVSVSTFNLVAISLERYGAICRPLQSRVWQTKSHALKVIAATWCLSFTIMTPYPIYSNLVPFTKNNNQTANMCRFLLPSDAMQQSWQTFLLLILFLLPGIVMVVAYGLISLELYQGIKFDASQKKSAKEKKPSTGSSTRYEDSDGCYLQKSRPPRKLELQQLSSGSGGSRLNRIRSSSSAANLIAKKRVIRMLIVIVVLFFLCWMPIFSANAWRAYDTVSAEKHLSGTPISFILLLSYTSSCVNPIIYCFMNKRFRLGFMATFPCCPNPGPPGVRGEVGEEEDGRTIRALLSRYSYSHMSTSAPPP
Inhibitor
Name:
BDBM50290396
Synonyms:
1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl]-5-(2-fluoro-phenyl)-9-methyl-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl]-3-m-tolyl-urea | CHEMBL299800
Type:
Small organic molecule
Emp. Form.:
C34H36FN5O3
Mol. Mass.:
581.6797
SMILES:
Cc1cccc(NC(=O)NC2N=C(c3ccccc3F)c3cccc(C)c3N(CC(=O)N3CC4CCC(CC4)C3)C2=O)c1 |t:11,(9.11,-4.37,;8.34,-5.7,;9.11,-7.02,;8.37,-8.34,;6.84,-8.37,;6.07,-7.02,;4.53,-7.02,;3.22,-7.79,;3.22,-9.35,;1.87,-7.06,;.52,-7.82,;-.13,-9.24,;-1.62,-9.58,;-1.97,-11.08,;-3.45,-11.55,;-3.79,-13.05,;-2.65,-14.11,;-1.18,-13.62,;-.83,-12.13,;.64,-11.66,;-2.84,-8.63,;-4.19,-9.4,;-5.54,-8.63,;-5.54,-7.09,;-4.19,-6.29,;-4.2,-4.75,;-2.84,-7.06,;-1.72,-6.04,;-2.35,-4.62,;-1.45,-3.36,;.08,-3.53,;-2.07,-1.97,;-1.09,-.8,;-1.4,.71,;-2.84,.39,;-3.05,-1.1,;-4.54,-.71,;-4.16,.77,;-2.77,1.41,;-3.61,-1.93,;-.16,-6.44,;.82,-5.23,;6.83,-5.72,)|