Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Leukotriene A-4 hydrolase
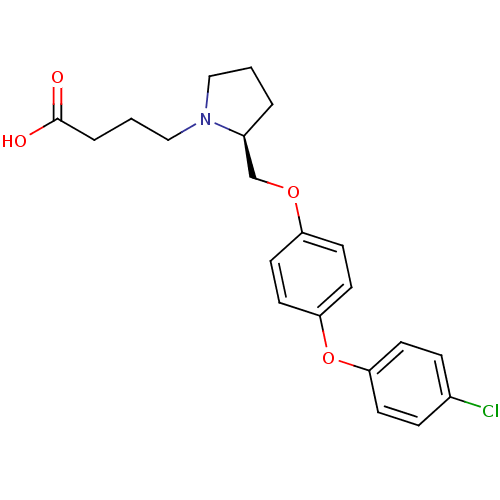
Ligand
BDBM50303649
Substrate
n/a
Meas. Tech.
ChEMBL_596630 (CHEMBL1046157)
Kd
25±n/a nM
Citation
 Sandanayaka, V; Mamat, B; Mishra, RK; Winger, J; Krohn, M; Zhou, LM; Keyvan, M; Enache, L; Sullins, D; Onua, E; Zhang, J; Halldorsdottir, G; Sigthorsdottir, H; Thorlaksdottir, A; Sigthorsson, G; Thorsteinnsdottir, M; Davies, DR; Stewart, LJ; Zembower, DE; Andresson, T; Kiselyov, AS; Singh, J; Gurney, ME Discovery of 4-[(2S)-2-{[4-(4-chlorophenoxy)phenoxy]methyl}-1-pyrrolidinyl]butanoic acid (DG-051) as a novel leukotriene A4 hydrolase inhibitor of leukotriene B4 biosynthesis. J Med Chem 53:573-85 (2010) [PubMed] Article
Sandanayaka, V; Mamat, B; Mishra, RK; Winger, J; Krohn, M; Zhou, LM; Keyvan, M; Enache, L; Sullins, D; Onua, E; Zhang, J; Halldorsdottir, G; Sigthorsdottir, H; Thorlaksdottir, A; Sigthorsson, G; Thorsteinnsdottir, M; Davies, DR; Stewart, LJ; Zembower, DE; Andresson, T; Kiselyov, AS; Singh, J; Gurney, ME Discovery of 4-[(2S)-2-{[4-(4-chlorophenoxy)phenoxy]methyl}-1-pyrrolidinyl]butanoic acid (DG-051) as a novel leukotriene A4 hydrolase inhibitor of leukotriene B4 biosynthesis. J Med Chem 53:573-85 (2010) [PubMed] Article More Info.:
Target
Name:
Leukotriene A-4 hydrolase
Synonyms:
LKHA4_HUMAN | LTA-4 hydrolase | LTA4 | LTA4H | Leukotriene A(4) hydrolase | Leukotriene A-4 hydrolase (LTA4H) | Leukotriene A4 hydrolase
Type:
Hydrolase; metalloprotease
Mol. Mass.:
69280.41
Organism:
Homo sapiens (Human)
Description:
Human recombinant LTA4H.
Residue:
611
Sequence:
MPEIVDTCSLASPASVCRTKHLHLRCSVDFTRRTLTGTAALTVQSQEDNLRSLVLDTKDLTIEKVVINGQEVKYALGERQSYKGSPMEISLPIALSKNQEIVIEISFETSPKSSALQWLTPEQTSGKEHPYLFSQCQAIHCRAILPCQDTPSVKLTYTAEVSVPKELVALMSAIRDGETPDPEDPSRKIYKFIQKVPIPCYLIALVVGALESRQIGPRTLVWSEKEQVEKSAYEFSETESMLKIAEDLGGPYVWGQYDLLVLPPSFPYGGMENPCLTFVTPTLLAGDKSLSNVIAHEISHSWTGNLVTNKTWDHFWLNEGHTVYLERHICGRLFGEKFRHFNALGGWGELQNSVKTFGETHPFTKLVVDLTDIDPDVAYSSVPYEKGFALLFYLEQLLGGPEIFLGFLKAYVEKFSYKSITTDDWKDFLYSYFKDKVDVLNQVDWNAWLYSPGLPPIKPNYDMTLTNACIALSQRWITAKEDDLNSFNATDLKDLSSHQLNEFLAQTLQRAPLPLGHIKRMQEVYNFNAINNSEIRFRWLRLCIQSKWEDAIPLALKMATEQGRMKFTRPLFKDLAAFDKSHDQAVRTYQEHKASMHPVTAMLVGKDLKVD