Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 1A2
Ligand
BDBM50318220
Substrate
n/a
Meas. Tech.
ChEMBL_629054 (CHEMBL1121012)
IC50
>35000±n/a nM
Citation
 Watterson, SH; Xiao, Z; Dodd, DS; Tortolani, DR; Vaccaro, W; Potin, D; Launay, M; Stetsko, DK; Skala, S; Davis, PM; Lee, D; Yang, X; McIntyre, KW; Balimane, P; Patel, K; Yang, Z; Marathe, P; Kadiyala, P; Tebben, AJ; Sheriff, S; Chang, CY; Ziemba, T; Zhang, H; Chen, BC; DelMonte, AJ; Aranibar, N; McKinnon, M; Barrish, JC; Suchard, SJ; Murali Dhar, TG Small molecule antagonist of leukocyte function associated antigen-1 (LFA-1): structure-activity relationships leading to the identification of 6-((5S,9R)-9-(4-cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]nonan-7-yl)nicotinic acid (BMS-688521). J Med Chem 53:3814-30 (2010) [PubMed] Article
Watterson, SH; Xiao, Z; Dodd, DS; Tortolani, DR; Vaccaro, W; Potin, D; Launay, M; Stetsko, DK; Skala, S; Davis, PM; Lee, D; Yang, X; McIntyre, KW; Balimane, P; Patel, K; Yang, Z; Marathe, P; Kadiyala, P; Tebben, AJ; Sheriff, S; Chang, CY; Ziemba, T; Zhang, H; Chen, BC; DelMonte, AJ; Aranibar, N; McKinnon, M; Barrish, JC; Suchard, SJ; Murali Dhar, TG Small molecule antagonist of leukocyte function associated antigen-1 (LFA-1): structure-activity relationships leading to the identification of 6-((5S,9R)-9-(4-cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]nonan-7-yl)nicotinic acid (BMS-688521). J Med Chem 53:3814-30 (2010) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 1A2
Synonyms:
CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3
Type:
Enzyme
Mol. Mass.:
58423.38
Organism:
Homo sapiens (Human)
Description:
P05177
Residue:
516
Sequence:
MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKNPHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDGQSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELMAGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFPILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGNLIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLSDRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPELWEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLEFSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
Inhibitor
Name:
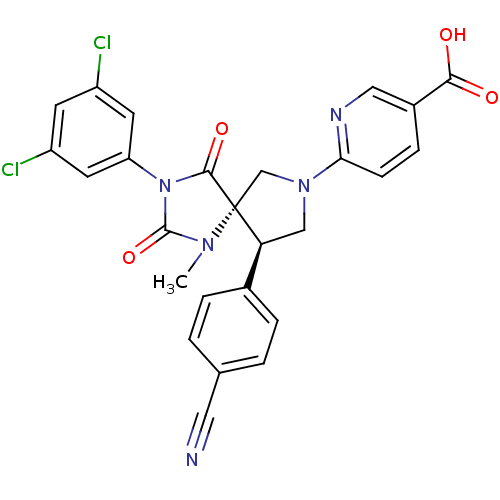
BDBM50318220
Synonyms:
6-((5S,9R)-9-(4-Cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]nonan-7-yl)nicotinic Acid | 6-[(5S,9R)-9-(4-cyanophenyl)-3-(3,5-dichlorophenyl)-1-methyl-2,4-dioxo-1,3,7-triazaspiro[4.4]non-7-yl]pyridine-3-carboxylic acid | CHEMBL1098726
Type:
Small organic molecule
Emp. Form.:
C26H19Cl2N5O4
Mol. Mass.:
536.366
SMILES:
CN1C(=O)N(C(=O)[C@]11CN(C[C@H]1c1ccc(cc1)C#N)c1ccc(cn1)C(O)=O)c1cc(Cl)cc(Cl)c1 |r|