Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Sodium/potassium-transporting ATPase subunit alpha-1
Ligand
BDBM46355
Substrate
n/a
Meas. Tech.
ChEMBL_144092 (CHEMBL857543)
IC50
500±n/a nM
Citation
 Cerri, A; Serra, F; Ferrari, P; Folpini, E; Padoani, G; Melloni, P Synthesis, cardiotonic activity, and structure-activity relationships of 17 beta-guanylhydrazone derivatives of 5 beta-androstane-3 beta, 14 beta-diol acting on the Na+,K(+)-ATPase receptor. J Med Chem 40:3484-8 (1997) [PubMed] Article
Cerri, A; Serra, F; Ferrari, P; Folpini, E; Padoani, G; Melloni, P Synthesis, cardiotonic activity, and structure-activity relationships of 17 beta-guanylhydrazone derivatives of 5 beta-androstane-3 beta, 14 beta-diol acting on the Na+,K(+)-ATPase receptor. J Med Chem 40:3484-8 (1997) [PubMed] Article More Info.:
Target
Name:
Sodium/potassium-transporting ATPase subunit alpha-1
Synonyms:
AT1A1_CANLF | ATP1A1 | Sodium/potassium-transporting ATPase alpha-1 chain
Type:
PROTEIN
Mol. Mass.:
112656.60
Organism:
Canis familiaris
Description:
ChEMBL_144092
Residue:
1021
Sequence:
MGKGVGRDKYEPAAVSEHGDKKKAKKERDMDELKKEVSMDDHKLSLDELHRKYGTDLSRGLTTARAAEILARDGPNALTPPPTTPEWVKFCRQLFGGFSMLLWIGAILCFLAYGIQAATEEEPQNDNLYLGVVLSAVVIITGCFSYYQEAKSSKIMESFKNMVPQQALVIRNGEKMSINAEEVVIGDLVEVKGGDRIPADLRIISANGCKVDNSSLTGESEPQTRSPDFTNENPLETRNIAFFSTNCVKGTARGIVVYTGDRTVMGRIATLASGLEGGQTPIAAEIEHFIHIITGVAVFLGVSFFILSLILEYTWLEAVIFLIGIIVANVPEGLLATVTVCLTLTAKRMARKNCLVKNLEAVETLGSTSTICSDKTGTLTQNRMTVAHMWFDNQIHEADTTENQSGVSFDKSSATWLALSRIAGLCNRAVFQANQENLPILKRAVAGDASESALLKCIELCCGSVKEMRDRYAKIVEIPFNSTNKYQLSIHKNPNTSEPRHLLVMKGAPERILDRCSSILLHGKEQPLDEELKDALQNAYLELGGLGERVLGFRHLFLPDEQFPEGFQFDTDDVNFPVENLCFVGFISMIGPPRAAVPDAVGKCRGAGIKVIMVTGDHPITAKAIAKGAGIISEGNETVEDIAARLNIPVRQVNPRDAKACVVHGSDLKDMTSEQLDGILKYHTEIVFARTSPQQKLIIVEGCQRQGAIVAVTGDGVNDSPALKKADIGVAMGIVGSDASKQAADMILLDDNFASIVTGVEEGRLIFDNLKKSIAYTLTSNIPEITPFLIFIIANIPLPLGTVTILCIDLGTDMVPAISLAYEQAESDIMKRQPRNPKTDKLVNERLISMAYGQIGMIQALGGFFTYFVILAENGFLPTHLLGLRVDWDDRWINDVEDSYGQQWTYEQRKIVEFTCHTAFFVSIVVVQWADLVICKTRRNSVFQQGMKNKILIFGLFEETALAAFLSYCPGMGVALRMYPLKPTWWFCAFPYSLLIFVYDEVRKLIIRRRPGGWVEKETYY
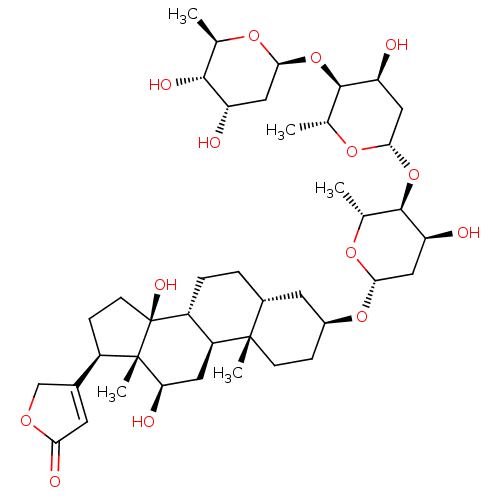
Inhibitor
Name:
BDBM46355
Synonyms:
DIGOXIN | MLS000069819 | SMR000059217 | US10668094, Compound Digoxin | cid_2724385
Type:
Small organic molecule
Emp. Form.:
C41H64O14
Mol. Mass.:
780.9385
SMILES:
C[C@H]1O[C@H](C[C@H](O)[C@@H]1O)O[C@H]1[C@@H](O)C[C@H](O[C@H]2[C@@H](O)C[C@H](O[C@H]3CC[C@@]4(C)[C@H](CC[C@@H]5[C@@H]4C[C@@H](O)[C@]4(C)[C@H](CC[C@]54O)C4=CC(=O)OC4)C3)O[C@@H]2C)O[C@@H]1C |t:46|