Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Prothrombin
Ligand
BDBM50374345
Substrate
n/a
Meas. Tech.
ChEMBL_469216 (CHEMBL948917)
Ki
0.2±n/a nM
Citation
 Maryanoff, BE; Costanzo, MJ Inhibitors of proteases and amide hydrolases that employ an alpha-ketoheterocycle as a key enabling functionality. Bioorg Med Chem 16:1562-95 (2008) [PubMed] Article
Maryanoff, BE; Costanzo, MJ Inhibitors of proteases and amide hydrolases that employ an alpha-ketoheterocycle as a key enabling functionality. Bioorg Med Chem 16:1562-95 (2008) [PubMed] Article More Info.:
Target
Name:
Prothrombin
Synonyms:
Activation peptide fragment 1 | Activation peptide fragment 2 | Coagulation factor II | F2 | Prothrombin precursor | THRB_HUMAN | Thrombin heavy chain | Thrombin light chain
Type:
Protein
Mol. Mass.:
70029.57
Organism:
Homo sapiens (Human)
Description:
P00734
Residue:
622
Sequence:
MAHVRGLQLPGCLALAALCSLVHSQHVFLAPQQARSLLQRVRRANTFLEEVRKGNLERECVEETCSYEEAFEALESSTATDVFWAKYTACETARTPRDKLAACLEGNCAEGLGTNYRGHVNITRSGIECQLWRSRYPHKPEINSTTHPGADLQENFCRNPDSSTTGPWCYTTDPTVRRQECSIPVCGQDQVTVAMTPRSEGSSVNLSPPLEQCVPDRGQQYQGRLAVTTHGLPCLAWASAQAKALSKHQDFNSAVQLVENFCRNPDGDEEGVWCYVAGKPGDFGYCDLNYCEEAVEEETGDGLDEDSDRAIEGRTATSEYQTFFNPRTFGSGEADCGLRPLFEKKSLEDKTERELLESYIDGRIVEGSDAEIGMSPWQVMLFRKSPQELLCGASLISDRWVLTAAHCLLYPPWDKNFTENDLLVRIGKHSRTRYERNIEKISMLEKIYIHPRYNWRENLDRDIALMKLKKPVAFSDYIHPVCLPDRETAASLLQAGYKGRVTGWGNLKETWTANVGKGQPSVLQVVNLPIVERPVCKDSTRIRITDNMFCAGYKPDEGKRGDACEGDSGGPFVMKSPFNNRWYQMGIVSWGEGCDRDGKYGFYTHVFRLKKWIQKVIDQFGE
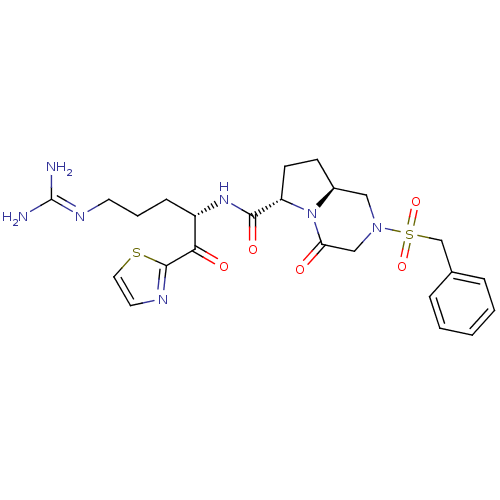
Inhibitor
Name:
BDBM50374345
Synonyms:
CHEMBL256253
Type:
Small organic molecule
Emp. Form.:
C24H31N7O5S2
Mol. Mass.:
561.677
SMILES:
[#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6@H]-2-[#6]-[#7](-[#6]-[#6](=O)-[#7]-1-2)S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-c1nccs1