Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cholinesterase
Ligand
BDBM50298403
Substrate
n/a
Meas. Tech.
ChEMBL_588497 (CHEMBL1041237)
IC50
3640±n/a nM
Citation
 Ronco, C; Sorin, G; Nachon, F; Foucault, R; Jean, L; Romieu, A; Renard, PY Synthesis and structure-activity relationship of Huprine derivatives as human acetylcholinesterase inhibitors. Bioorg Med Chem 17:4523-36 (2009) [PubMed] Article
Ronco, C; Sorin, G; Nachon, F; Foucault, R; Jean, L; Romieu, A; Renard, PY Synthesis and structure-activity relationship of Huprine derivatives as human acetylcholinesterase inhibitors. Bioorg Med Chem 17:4523-36 (2009) [PubMed] Article More Info.:
Target
Name:
Cholinesterase
Synonyms:
Acylcholine acylhydrolase | BCHE | Butyrylcholine esterase (BChE) | Butyrylcholinesterase (BChE) | Butyrylcholinesterase (BuChE) | CHE1 | CHLE_HUMAN | Choline esterase II | Cholinesterases | Cholinesterases; ACHE & BCHE | Pseudocholinesterase
Type:
Homotetramer
Mol. Mass.:
68422.27
Organism:
Homo sapiens (Human)
Description:
P06276
Residue:
602
Sequence:
MHSKVTIICIRFLFWFLLLCMLIGKSHTEDDIIIATKNGKVRGMNLTVFGGTVTAFLGIPYAQPPLGRLRFKKPQSLTKWSDIWNATKYANSCCQNIDQSFPGFHGSEMWNPNTDLSEDCLYLNVWIPAPKPKNATVLIWIYGGGFQTGTSSLHVYDGKFLARVERVIVVSMNYRVGALGFLALPGNPEAPGNMGLFDQQLALQWVQKNIAAFGGNPKSVTLFGESAGAASVSLHLLSPGSHSLFTRAILQSGSFNAPWAVTSLYEARNRTLNLAKLTGCSRENETEIIKCLRNKDPQEILLNEAFVVPYGTPLSVNFGPTVDGDFLTDMPDILLELGQFKKTQILVGVNKDEGTAFLVYGAPGFSKDNNSIITRKEFQEGLKIFFPGVSEFGKESILFHYTDWVDDQRPENYREALGDVVGDYNFICPALEFTKKFSEWGNNAFFYYFEHRSSKLPWPEWMGVMHGYEIEFVFGLPLERRDNYTKAEEILSRSIVKRWANFAKYGNPNETQNNSTSWPVFKSTEQKYLTLNTESTRIMTKLRAQQCRFWTSFFPKVLEMTGNIDEAEWEWKAGFHRWNNYMMDWKNQFNDYTSKKESCVGL
Inhibitor
Name:
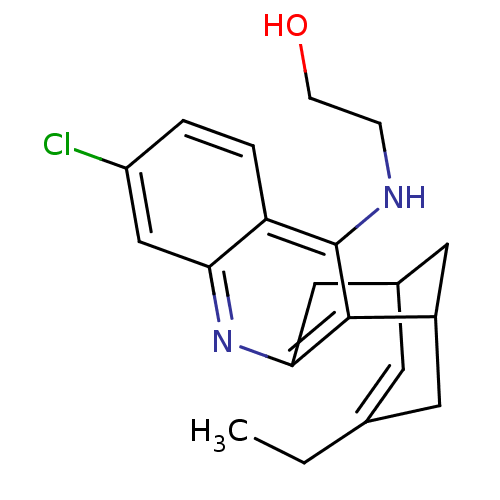
BDBM50298403
Synonyms:
(+/-)-N-(2-Hydroxyethyl)-3-chloro-6,7,10,11-tetrahydro-12-ylamino-9-ethyl-7,11-methanocyclooctan[b]-quinoline | CHEMBL573095
Type:
Small organic molecule
Emp. Form.:
C20H23ClN2O
Mol. Mass.:
342.862
SMILES:
CCC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCO |t:2,TLB:19:8:5:7.3.2,THB:1:2:5:10.9.8,11:9:5:7.3.2|