Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cholecystokinin receptor type A
Ligand
BDBM50321606
Substrate
n/a
Meas. Tech.
ChEMBL_639788 (CHEMBL1175733)
Ki
18±n/a nM
Citation
 Lee, YS; Fernandes, S; Kulkarani, V; Mayorov, A; Davis, P; Ma, SW; Brown, K; Gillies, RJ; Lai, J; Porreca, F; Hruby, VJ Design and synthesis of trivalent ligands targeting opioid, cholecystokinin, and melanocortin receptors for the treatment of pain. Bioorg Med Chem Lett 20:4080-4 (2010) [PubMed] Article
Lee, YS; Fernandes, S; Kulkarani, V; Mayorov, A; Davis, P; Ma, SW; Brown, K; Gillies, RJ; Lai, J; Porreca, F; Hruby, VJ Design and synthesis of trivalent ligands targeting opioid, cholecystokinin, and melanocortin receptors for the treatment of pain. Bioorg Med Chem Lett 20:4080-4 (2010) [PubMed] Article More Info.:
Target
Name:
Cholecystokinin receptor type A
Synonyms:
CCK-A receptor | CCK-AR | CCK1-R | CCKAR | CCKAR_HUMAN | CCKRA | Cholecystokinin receptor | Cholecystokinin receptor type A | Cholecystokinin-1 Receptor
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
47859.34
Organism:
Homo sapiens (Human)
Description:
Stable expression of human CCK-1 receptors in HEK 293 cells.
Residue:
428
Sequence:
MDVVDSLLVNGSNITPPCELGLENETLFCLDQPRPSKEWQPAVQILLYSLIFLLSVLGNTLVITVLIRNKRMRTVTNIFLLSLAVSDLMLCLFCMPFNLIPNLLKDFIFGSAVCKTTTYFMGTSVSVSTFNLVAISLERYGAICKPLQSRVWQTKSHALKVIAATWCLSFTIMTPYPIYSNLVPFTKNNNQTANMCRFLLPNDVMQQSWHTFLLLILFLIPGIVMMVAYGLISLELYQGIKFEASQKKSAKERKPSTTSSGKYEDSDGCYLQKTRPPRKLELRQLSTGSSSRANRIRSNSSAANLMAKKRVIRMLIVIVVLFFLCWMPIFSANAWRAYDTASAERRLSGTPISFILLLSYTSSCVNPIIYCFMNKRFRLGFMATFPCCPNPGPPGARGEVGEEEEGGTTGASLSRFSYSHMSASVPPQ
Inhibitor
Name:
BDBM50321606
Synonyms:
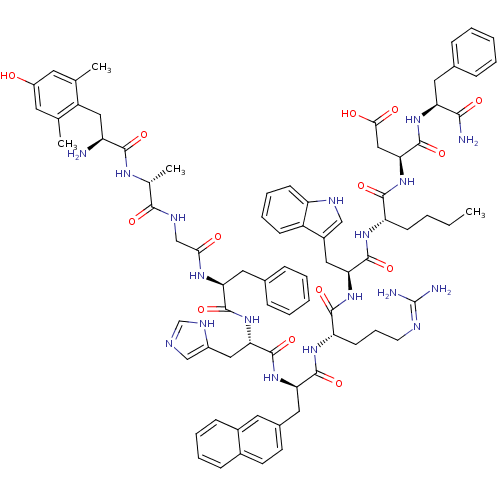
(3S,6S,9S,12S,15R,18S,21S,27R,30S)-18-((1H-imidazol-5-yl)methyl)-9-((1H-indol-3-yl)methyl)-30-amino-3-((S)-1-amino-1-oxo-3-phenylpropan-2-ylcarbamoyl)-21-benzyl-6-butyl-12-(3-guanidinopropyl)-31-(4-hydroxy-2,6-dimethylphenyl)-27-methyl-15-(naphthalen-2-ylmethyl)-5,8,11,14,17,20,23,26,29-nonaoxo-4,7,10,13,16,19,22,25,28-nonaazahentriacontan-1-oic acid | CHEMBL1172253
Type:
Small organic molecule
Emp. Form.:
C80H98N18O14
Mol. Mass.:
1535.7463
SMILES:
CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc1ccc2ccccc2c1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)[C@@H](C)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r,wU:8.20,58.71,93.99,78.84,wD:73.79,48.59,22.31,33.48,4.4,101.107,(24.59,-5.48,;25.93,-4.72,;25.93,-3.18,;24.59,-2.4,;24.59,-.86,;23.26,-.1,;21.93,-.87,;21.93,-2.41,;20.59,-.11,;20.59,1.44,;22,2.07,;23.25,1.19,;24.48,2.12,;23.98,3.58,;24.73,4.92,;23.94,6.24,;22.4,6.22,;21.65,4.87,;22.44,3.55,;19.25,-.87,;17.91,-.12,;17.91,1.43,;16.57,-.89,;16.57,-2.43,;17.91,-3.2,;17.91,-4.73,;19.24,-5.51,;19.24,-7.05,;17.93,-7.8,;20.61,-7.81,;15.25,-.1,;13.92,-.87,;13.92,-2.41,;12.59,-.1,;12.6,1.44,;13.92,2.21,;15.26,1.44,;16.59,2.21,;16.6,3.75,;17.93,4.53,;17.92,6.07,;16.58,6.84,;15.25,6.06,;15.26,4.52,;13.92,3.75,;11.26,-.86,;9.92,-.1,;9.92,1.45,;8.58,-.87,;8.58,-2.41,;9.92,-3.18,;11.32,-2.55,;12.36,-3.69,;11.59,-5.03,;10.08,-4.71,;7.25,-.09,;5.91,-.85,;5.91,-2.39,;4.58,-.08,;4.58,1.46,;5.91,2.23,;7.25,1.47,;8.58,2.24,;8.58,3.78,;7.24,4.55,;5.91,3.78,;3.25,-.85,;1.92,-.07,;1.92,1.47,;.58,-.85,;-.75,-.07,;-2.08,-.85,;-2.08,-2.38,;-3.41,-.07,;-3.41,1.47,;-4.75,-.84,;-6.09,-.08,;-6.09,1.47,;-7.42,-.86,;-7.4,-2.4,;-8.75,-.1,;-10.08,-.88,;-10.06,-2.42,;-8.72,-3.17,;-11.4,-3.2,;-12.73,-2.44,;-14.07,-3.22,;-12.76,-.9,;-11.41,-.13,;-11.43,1.41,;25.93,-.09,;25.93,1.45,;27.26,-.87,;28.59,-.09,;28.59,1.45,;29.93,2.21,;31.25,1.45,;29.93,3.76,;29.93,-.87,;29.93,-2.4,;31.26,-.1,;32.6,-.87,;32.6,-2.4,;33.93,-3.17,;35.28,-2.4,;36.61,-3.17,;36.61,-4.71,;35.27,-5.48,;33.94,-4.71,;33.93,-.1,;35.27,-.86,;33.93,1.45,)|