Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Receptor-type tyrosine-protein phosphatase F
Ligand
BDBM50148911
Substrate
n/a
Meas. Tech.
ChEMBL_848287 (CHEMBL2149376)
IC50
3800±n/a nM
Citation
 Ramírez-Espinosa, JJ; Rios, MY; López-Martínez, S; López-Vallejo, F; Medina-Franco, JL; Paoli, P; Camici, G; Navarrete-Vázquez, G; Ortiz-Andrade, R; Estrada-Soto, S Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTP-1B: in vitro, in silico, and in vivo approaches. Eur J Med Chem 46:2243-51 (2011) [PubMed] Article
Ramírez-Espinosa, JJ; Rios, MY; López-Martínez, S; López-Vallejo, F; Medina-Franco, JL; Paoli, P; Camici, G; Navarrete-Vázquez, G; Ortiz-Andrade, R; Estrada-Soto, S Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTP-1B: in vitro, in silico, and in vivo approaches. Eur J Med Chem 46:2243-51 (2011) [PubMed] Article More Info.:
Target
Name:
Receptor-type tyrosine-protein phosphatase F
Synonyms:
LAR | Leukocyte common antigen related | Leukocyte common antigen related (LAR) | PTPRF | PTPRF_HUMAN | Receptor-type tyrosine-protein phosphatase F | Receptor-type tyrosine-protein phosphatase F (LAR)
Type:
Protein
Mol. Mass.:
212869.85
Organism:
Homo sapiens (Human)
Description:
P10586
Residue:
1907
Sequence:
MAPEPAPGRTMVPLVPALVMLGLVAGAHGDSKPVFIKVPEDQTGLSGGVASFVCQATGEPKPRITWMKKGKKVSSQRFEVIEFDDGAGSVLRIQPLRVQRDEAIYECTATNSLGEINTSAKLSVLEEEQLPPGFPSIDMGPQLKVVEKARTATMLCAAGGNPDPEISWFKDFLPVDPATSNGRIKQLRSGALQIESSEESDQGKYECVATNSAGTRYSAPANLYVRVRRVAPRFSIPPSSQEVMPGGSVNLTCVAVGAPMPYVKWMMGAEELTKEDEMPVGRNVLELSNVVRSANYTCVAISSLGMIEATAQVTVKALPKPPIDLVVTETTATSVTLTWDSGNSEPVTYYGIQYRAAGTEGPFQEVDGVATTRYSIGGLSPFSEYAFRVLAVNSIGRGPPSEAVRARTGEQAPSSPPRRVQARMLSASTMLVQWEPPEEPNGLVRGYRVYYTPDSRRPPNAWHKHNTDAGLLTTVGSLLPGITYSLRVLAFTAVGDGPPSPTIQVKTQQGVPAQPADFQAEVESDTRIQLSWLLPPQERIIMYELVYWAAEDEDQQHKVTFDPTSSYTLEDLKPDTLYRFQLAARSDMGVGVFTPTIEARTAQSTPSAPPQKVMCVSMGSTTVRVSWVPPPADSRNGVITQYSVAYEAVDGEDRGRHVVDGISREHSSWDLVGLEKWTEYRVWVRAHTDVGPGPESSPVLVRTDEDVPSGPPRKVEVEPLNSTAVHVYWKLPVPSKQHGQIRGYQVTYVRLENGEPRGLPIIQDVMLAEAQWRPEESEDYETTISGLTPETTYSVTVAAYTTKGDGARSKPKIVTTTGAVPGRPTMMISTTAMNTALLQWHPPKELPGELLGYRLQYCRADEARPNTIDFGKDDQHFTVTGLHKGTTYIFRLAAKNRAGLGEEFEKEIRTPEDLPSGFPQNLHVTGLTTSTTELAWDPPVLAERNGRIISYTVVFRDINSQQELQNITTDTRFTLTGLKPDTTYDIKVRAWTSKGSGPLSPSIQSRTMPVEQVFAKNFRVAAAMKTSVLLSWEVPDSYKSAVPFKILYNGQSVEVDGHSMRKLIADLQPNTEYSFVLMNRGSSAGGLQHLVSIRTAPDLLPHKPLPASAYIEDGRFDLSMPHVQDPSLVRWFYIVVVPIDRVGGSMLTPRWSTPEELELDELLEAIEQGGEEQRRRRRQAERLKPYVAAQLDVLPETFTLGDKKNYRGFYNRPLSPDLSYQCFVLASLKEPMDQKRYASSPYSDEIVVQVTPAQQQEEPEMLWVTGPVLAVILIILIVIAILLFKRKRTHSPSSKDEQSIGLKDSLLAHSSDPVEMRRLNYQTPGMRDHPPIPITDLADNIERLKANDGLKFSQEYESIDPGQQFTWENSNLEVNKPKNRYANVIAYDHSRVILTSIDGVPGSDYINANYIDGYRKQNAYIATQGPLPETMGDFWRMVWEQRTATVVMMTRLEEKSRVKCDQYWPARGTETCGLIQVTLLDTVELATYTVRTFALHKSGSSEKRELRQFQFMAWPDHGVPEYPTPILAFLRRVKACNPLDAGPMVVHCSAGVGRTGCFIVIDAMLERMKHEKTVDIYGHVTCMRSQRNYMVQTEDQYVFIHEALLEAATCGHTEVPARNLYAHIQKLGQVPPGESVTAMELEFKLLASSKAHTSRFISANLPCNKFKNRLVNIMPYELTRVCLQPIRGVEGSDYINASFLDGYRQQKAYIATQGPLAESTEDFWRMLWEHNSTIIVMLTKLREMGREKCHQYWPAERSARYQYFVVDPMAEYNMPQYILREFKVTDARDGQSRTIRQFQFTDWPEQGVPKTGEGFIDFIGQVHKTKEQFGQDGPITVHCSAGVGRTGVFITLSIVLERMRYEGVVDMFQTVKTLRTQRPAMVQTEDQYQLCYRAALEYLGSFDHYAT
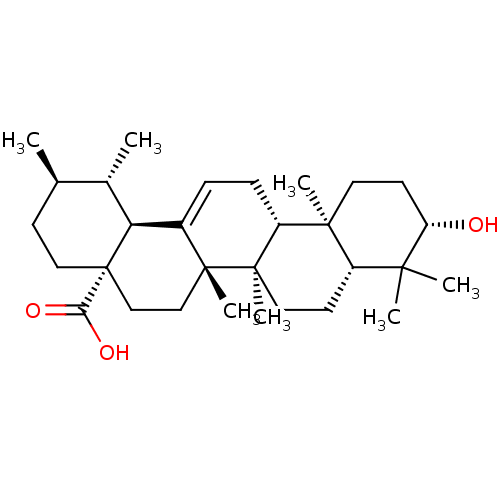
Inhibitor
Name:
BDBM50148911
Synonyms:
(3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hydroxyurs-12-en-28-oic acid | CHEMBL169 | Ursolic acid | malol | prunol | urson
Type:
Small organic molecule
Emp. Form.:
C30H48O3
Mol. Mass.:
456.7003
SMILES:
C[C@@H]1CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2[C@H]1C)C(O)=O |r,c:9|