Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Urotensin-2 receptor
Ligand
BDBM86516
Substrate
n/a
Ki
1.2±n/a nM
Comments
PDSP_3691
Citation
 Clozel, M; Binkert, C; Birker-Robaczewska, M; Boukhadra, C; Ding, SS; Fischli, W; Hess, P; Mathys, B; Morrison, K; Müller, C; Müller, C; Nayler, O; Qiu, C; Rey, M; Scherz, MW; Velker, J; Weller, T; Xi, JF; Ziltener, P Pharmacology of the urotensin-II receptor antagonist palosuran (ACT-058362; 1-[2-(4-benzyl-4-hydroxy-piperidin-1-yl)-ethyl]-3-(2-methyl-quinolin-4-yl)-urea sulfate salt): first demonstration of a pathophysiological role of the urotensin System. J Pharmacol Exp Ther 311:204-12 (2004) [PubMed] Article
Clozel, M; Binkert, C; Birker-Robaczewska, M; Boukhadra, C; Ding, SS; Fischli, W; Hess, P; Mathys, B; Morrison, K; Müller, C; Müller, C; Nayler, O; Qiu, C; Rey, M; Scherz, MW; Velker, J; Weller, T; Xi, JF; Ziltener, P Pharmacology of the urotensin-II receptor antagonist palosuran (ACT-058362; 1-[2-(4-benzyl-4-hydroxy-piperidin-1-yl)-ethyl]-3-(2-methyl-quinolin-4-yl)-urea sulfate salt): first demonstration of a pathophysiological role of the urotensin System. J Pharmacol Exp Ther 311:204-12 (2004) [PubMed] Article More Info.:
Target
Name:
Urotensin-2 receptor
Synonyms:
G-protein coupled receptor 14 | GPR14 | UR-II-R | UR2R_HUMAN | UTS2R | Urotensin II receptor | Urotensin-II
Type:
Enzyme Catalytic Domain
Mol. Mass.:
42159.71
Organism:
Homo sapiens (Human)
Description:
Urotensin-II UTS2R HUMAN::Q9UKP6
Residue:
389
Sequence:
MALTPESPSSFPGLAATGSSVPEPPGGPNATLNSSWASPTEPSSLEDLVATGTIGTLLSAMGVVGVVGNAYTLVVTCRSLRAVASMYVYVVNLALADLLYLLSIPFIVATYVTKEWHFGDVGCRVLFGLDFLTMHASIFTLTVMSSERYAAVLRPLDTVQRPKGYRKLLALGTWLLALLLTLPVMLAMRLVRRGPKSLCLPAWGPRAHRAYLTLLFATSIAGPGLLIGLLYARLARAYRRSQRASFKRARRPGARALRLVLGIVLLFWACFLPFWLWQLLAQYHQAPLAPRTARIVNYLTTCLTYGNSCANPFLYTLLTRNYRDHLRGRVRGPGSGGGRGPVPSLQPRARFQRCSGRSLSSCSPQPTDSLVLAPAAPARPAPEGPRAPA
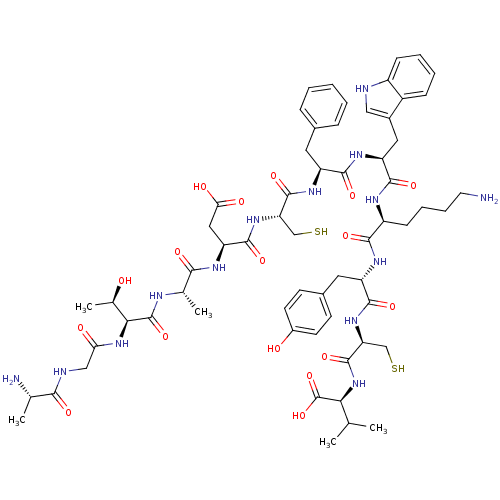
Inhibitor
Name:
BDBM86516
Synonyms:
ETPDCFWKYCV | Human U-II | L-Ala-Gly-L-Thr-L-Ala-L-Asp-L-Cys-L-Phe-L-Trp-L-Lys-L-Tyr-L-Cys-L-Val-OH | Urotensin II
Type:
Small organic molecule
Emp. Form.:
C62H86N14O17S2
Mol. Mass.:
1363.56
SMILES:
CC(C)[C@H](NC(=O)[C@H](CS)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CS)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O)C(O)=O