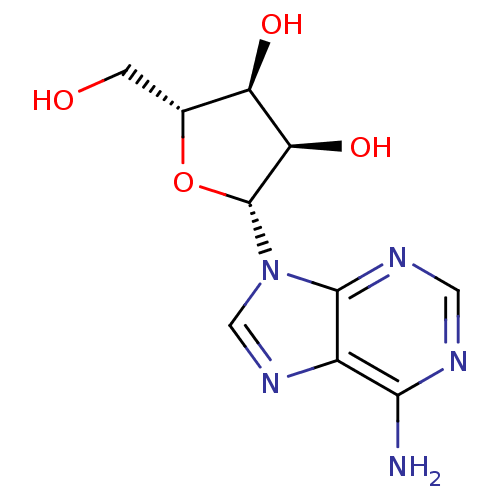
BDBM14487 (2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol::Adenine-beta-D-arabinofuranoside::Adenosine::CHEMBL477::N6-Methylado::[U-14C]adenosine::cid_191::cid_60961
SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O
InChI Key InChIKey=OIRDTQYFTABQOQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 112 hits for monomerid = 14487
Found 112 hits for monomerid = 14487
Affinity DataIC50: 1.10nMAssay Description:Agonist activity at human Adenosine A3 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Binding affinity for Adenosine A2 receptor in corpora striata of rats using [3H]NECAMore data for this Ligand-Target Pair
Affinity DataKi: 5.10nMAssay Description:Inhibition of [3H]-CHA binding to rat brain membrane Adenosine A1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Evaluated for the binding affinity towards the Adenosine A1 receptor in corpora striata of rats using [3H]CHA as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Displacement of [3H]R-PIA from rat brain membrane Adenosine A1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 13nMAssay Description:Agonist activity at human adenosine A3 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation incubated for...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Affinity for Adenosine A1 receptor determined by [3H]N6-cyclohexyladenosine binding to rat brain membranesMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of binding of [3H]N6-cyclohexyladenosine to adenosine A1 receptor of rat whole brain membranes.More data for this Ligand-Target Pair
Affinity DataKd: 17nMAssay Description:Binding affinity to human wild type adenosine A2A receptor expressed in Expi293F cells assessed as dissociation constant by surface plasmon resonance...More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Binding affinity to A2A adenosine receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 25nMAssay Description:Agonist activity at human adenosine A2A receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation incubated fo...More data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Displacement of specific [3H]-CGS- 21680 binding to adenosine A2A receptor in rat striatal membranesMore data for this Ligand-Target Pair
Affinity DataKi: 37nMAssay Description:Affinity for Adenosine A2 receptor determined by [3H]NECA binding to rat striatal membranesMore data for this Ligand-Target Pair
Affinity DataEC50: 39nMAssay Description:Agonist activity at human A1AR expressed in HEK293T/17 cells assessed as inhibition of isoproterenol-induced cAMP accumulation incubated for 10 mins ...More data for this Ligand-Target Pair
Affinity DataEC50: 51nMAssay Description:Concentration required for coronary arteries vasodilation at the A2 adenosine receptor in langendorff guinea pig heart preparationMore data for this Ligand-Target Pair
Affinity DataEC50: 52nMAssay Description:Agonist activity at human adenosine A2B receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation incubated fo...More data for this Ligand-Target Pair
Affinity DataEC50: 69nMAssay Description:Agonist activity at human adenosine A1 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation incubated for...More data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Displacement of [125I]- AB-MECA from rat adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 150nMAssay Description:Binding affinity to human wild type adenosine A2A receptor expressed in Expi293F cells assessed as inhibition constant by surface plasmon resonance a...More data for this Ligand-Target Pair
Affinity DataEC50: 290nMAssay Description:Agonist activity at human recombinant adenosine A3 receptor by cAMP assayMore data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Binding affinity to human wild type adenosine A3 receptor expressed in Expi293F cells assessed as inhibition constant by surface plasmon resonance as...More data for this Ligand-Target Pair
Affinity DataEC50: 290nMAssay Description:Agonist activity at human adenosine A3 receptor expressed in CHO cells assessed as increase of intracellular calcium levelMore data for this Ligand-Target Pair
Affinity DataEC50: 290nMAssay Description:Agonist activity at human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 290nMAssay Description:Agonist activity at human adenosine A3 receptor transfected in CHO cells assessed as changes in cAMP formation in the presence of nitrobenzylthioinos...More data for this Ligand-Target Pair
Affinity DataKd: 295nMAssay Description:Inhibition of CA200645 binding to NanoLuc-fused human adenosine A1 receptor expressed in HEK293 cells assessed as kinetic dissociation constant by Na...More data for this Ligand-Target Pair
Affinity DataEC50: 310nMAssay Description:Agonist activity at human recombinant adenosine A1 receptor by cAMP assayMore data for this Ligand-Target Pair
Affinity DataEC50: 310nMAssay Description:Agonist activity at human adenosine A1 receptor expressed in CHO cells assessed as increase of intracellular calcium levelMore data for this Ligand-Target Pair
Affinity DataEC50: 310nMAssay Description:Agonist activity at human adenosine A1 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 310nMAssay Description:Agonist activity at human adenosine A1 receptor transfected in CHO cells assessed as changes in cAMP formation in the presence of nitrobenzylthioinos...More data for this Ligand-Target Pair
Affinity DataEC50: 700nMAssay Description:Agonist activity at human adenosine A2A receptor expressed in CHO cells assessed as increase of intracellular calcium levelMore data for this Ligand-Target Pair
Affinity DataEC50: 700nMAssay Description:Agonist activity at human adenosine A2A receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 700nMAssay Description:Agonist activity at human recombinant adenosine receptor A2a by cAMP assayMore data for this Ligand-Target Pair
Affinity DataKi: 700nMAssay Description:Binding affinity to human recombinant adenosine receptor A2A expressed in CHO cells assessed as inhibitory constant by radioligand competition assayMore data for this Ligand-Target Pair
Affinity DataEC50: 730nMAssay Description:Agonist activity at human adenosine A2A receptor transfected in CHO cells assessed as changes in cAMP formation in the presence of nitrobenzylthioino...More data for this Ligand-Target Pair
Affinity DataKd: 740nMAssay Description:Binding affinity to human wild type adenosine A3 receptor expressed in Expi293F cells assessed as dissociation constant by surface plasmon resonance ...More data for this Ligand-Target Pair
Affinity DataKi: 813nMAssay Description:Inhibition of CA200645 binding to NanoLuc-fused human adenosine A1 receptor expressed in HEK293 cells measured for 10 mins by NanoBRET competition bi...More data for this Ligand-Target Pair
Affinity DataKi: 871nMAssay Description:Inhibition of CA200645 binding to NanoLuc-fused rat adenosine A1 receptor expressed in HEK293 cells measured for 10 mins by NanoBRET competition bind...More data for this Ligand-Target Pair
Affinity DataEC50: 1.04E+3nMAssay Description:Activity against human wild type adenosine A3 receptor expressed in COS7 cells as measured by accumulation of inositol phosphate by PLC assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.41E+3nMAssay Description:Agonist activity at human A1AR assessed as induction of Ca2+ ion mobilization by orthogonal functional assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.80E+3nMAssay Description:Binding affinity to recombinant human CNT1 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 15 mins by sc...More data for this Ligand-Target Pair
Affinity DataKd: 1.95E+3nMAssay Description:Inhibition of CA200645 binding to NanoLuc-fused rat adenosine A1 receptor expressed in HEK293 cells assessed as kinetic dissociation constant by Nano...More data for this Ligand-Target Pair
Affinity DataKi: 2.10E+3nMAssay Description:Binding affinity to recombinant human CNT3 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 5 mins by sci...More data for this Ligand-Target Pair
Affinity DataEC50: 2.51E+3nMAssay Description:Agonist activity at human Adenosine A1 receptor expressed in yeast cells coexpressed with chimeric GPA1/Galphai1 after 16 hrs by beta galactosidase r...More data for this Ligand-Target Pair
TargetHeat shock protein HSP 90-alpha(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataKi: >3.00E+3nMAssay Description:Displacement of FITC-geldanamycin from HSP90alpha (unknown origin) after 24 hrs by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+3nMAssay Description:Binding affinity to recombinant human CNT3 expressed in Saccharomyces cerevisiae assessed as inhibition of [3H]-uridine transport after 5 mins by sci...More data for this Ligand-Target Pair
Affinity DataEC50: 3.39E+3nMAssay Description:Effective concentration required for prolongation of the stimulus-QRS interval by 50% of the maximum response at the A1 adenosine receptor in langend...More data for this Ligand-Target Pair
Affinity DataEC50: 3.39E+3nMAssay Description:Prolongation of the stimulus-QRS interval by 50% of the maximum response at the adenosine A1 receptor in langendorff guinea pig heart preparationMore data for this Ligand-Target Pair
Affinity DataKd: 4.40E+3nMAssay Description:Binding affinity to recombinant human biotinylated N-terminal GST-tagged autophosphorylated TAK1 (1 to 303 residues) fused with TAB1 (437 to 504 resi...More data for this Ligand-Target Pair