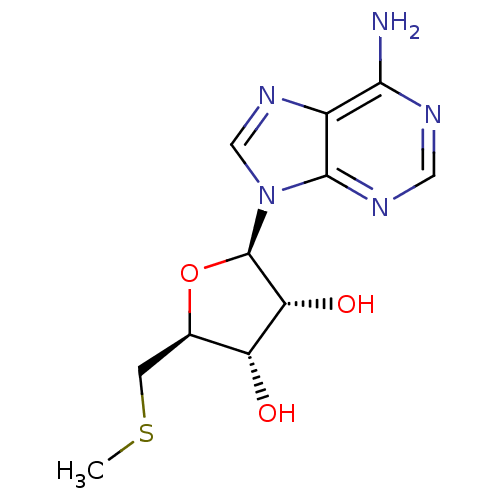
BDBM22111 (2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-[(methylsulfanyl)methyl]oxolane-3,4-diol::(2R,3R,4S,5S)-2-(6-aminopurin-9-yl)-5-(methylsulfanylmethyl)oxolane-3,4-diol::5'-Methylthioado::5-methylthioadenosine::CHEMBL277041::MTA
SMILES CSC[C@@H]1[C@H]([C@H]([C@@H](O1)n2cnc3c2ncnc3N)O)O
InChI Key InChIKey=WUUGFSXJNOTRMR-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 18 hits for monomerid = 22111
Found 18 hits for monomerid = 22111
Affinity DataKi: 150nMAssay Description:Displacement of [3H]CCPA from adenosine A1 receptor of rat cortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 210nMAssay Description:Displacement of [3H]DPCPX from adenosine A1 receptor of rat cortical membranesMore data for this Ligand-Target Pair
Affinity DataKi: 240nMAssay Description:Displacement of [3H]R-PIA from adenosine A1 receptor of rat cortical membranesMore data for this Ligand-Target Pair
TargetMethylosome protein 50/Protein arginine N-methyltransferase 5(Human)
Mirati Therapeutics
Curated by ChEMBL
Mirati Therapeutics
Curated by ChEMBL
Affinity DataKd: 661nMAssay Description:Inhibition of streptavidin sensor chip immobilized biotinylated human PRMT5/MEP50 assessed as dissociation constant by SPR analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 680nMAssay Description:Displacement of [3H]DPCPX from adenosine A1 receptor of rat cortical membranes without GTPMore data for this Ligand-Target Pair
Affinity DataKi: 680nMAssay Description:Displacement of [3H]PSB-11 from human adenosine A3 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Rat)
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by PDSP Ki Database
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by PDSP Ki Database
TargetAdenosine receptor A2a(Rat)
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by PDSP Ki Database
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by PDSP Ki Database
Affinity DataKi: 1.13E+3nMAssay Description:Displacement of [3H]-MSX-2 from Adenosine A2A receptor of rat striatal membranesMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Rat)
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by PDSP Ki Database
Warner-Lambert/Parke-Davis Pharmaceutical Research
Curated by PDSP Ki Database
Affinity DataKi: 1.18E+3nMAssay Description:Displacement of [3H]NECA from Adenosine A2A receptor of rat striatal membranesMore data for this Ligand-Target Pair
Affinity DataKi: 8.20E+3nMAssay Description:Binding affinity towards human adenosine A2B receptor in VA13 fibroblasts as inhibition of adenylate cyclase at 10 uM; Less than 10% inhibitionMore data for this Ligand-Target Pair
Affinity DataKi: 1.39E+4nMAssay Description:Displacement of [3H]PSB-298 from human adenosine A2B receptor expressed in HEK293 cells at 10 uM; Less than 10% inhibitionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.51E+4nMAssay Description:Displacement of [3H]DPCPX from adenosine A1 receptor of rat cortical membranes with GTPMore data for this Ligand-Target Pair
Affinity DataIC50: 7.10E+4nMAssay Description:Inhibition of forskolin-stimulated cAMP accumulation in chinese hamster ovary cell membranes expressing human adenosine A3 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.59E+5nMAssay Description:Inhibition of Plasmodium falciparum spermidine synthaseMore data for this Ligand-Target Pair
Affinity DataKi: 7.00E+5nMAssay Description:Inhibitory constant towards indole N-methyl-transferaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.50E+6nMAssay Description:Inhibition constant against Escherichia coli cyclopropane fatty acid synthaseMore data for this Ligand-Target Pair
Affinity DataKi: >5.40E+6nMAssay Description:Compound was tested for inhibition of Catechol O-methyltransferase using radiochemical assayMore data for this Ligand-Target Pair