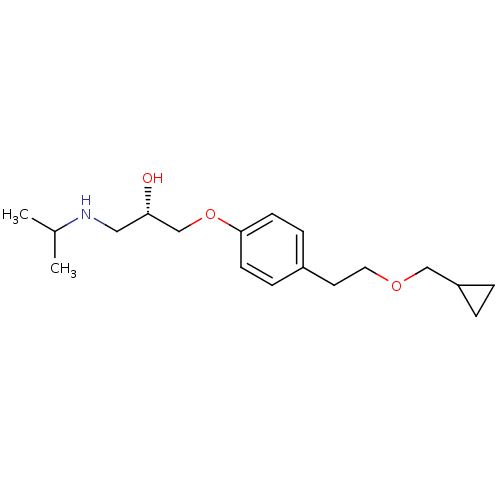
BDBM25752 Betaxolol::Levobetaxolol::[(2S)-3-{4-[2-(cyclopropylmethoxy)ethyl]phenoxy}-2-hydroxypropyl](propan-2-yl)amine
SMILES CC(C)NC[C@H](O)COc1ccc(CCOCC2CC2)cc1
InChI Key InChIKey=NWIUTZDMDHAVTP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 25752
Found 6 hits for monomerid = 25752
Affinity DataKd: 6.20nMpH: 7.4 T: 2°CAssay Description:The whole cell-binding studies were undertaken in CHO cell lines stably expressing each beta-adrenoceptor subtype. Nonspecific binding was determined...More data for this Ligand-Target Pair
Affinity DataKd: 42nMAssay Description:The whole cell-binding studies were undertaken in CHO cell lines stably expressing each beta-adrenoceptor subtype. Nonspecific binding was determined...More data for this Ligand-Target Pair
Affinity DataKd: 1.07E+3nMAssay Description:The whole cell-binding studies were undertaken in CHO cell lines stably expressing each beta-adrenoceptor subtype. Nonspecific binding was determined...More data for this Ligand-Target Pair