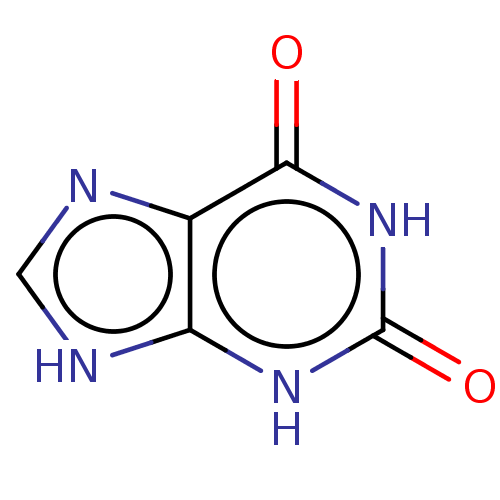
BDBM50227193 CHEBI:17712::Xanthine
SMILES O=c1[nH]c2[nH]cnc2c(=O)[nH]1
InChI Key InChIKey=LRFVTYWOQMYALW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50227193
Found 3 hits for monomerid = 50227193
Affinity DataKi: 1.30E+5nMAssay Description:Inhibition of adenosine stimulated accumulation of cyclic AMP at Adenosine A2 receptor of VA13 fibroblasts of ratMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of human BuChE using S-butyrylthiocholine iodide as substrate treated 5 mins before substrate addition measured up to 4 mins by Ellman's m...More data for this Ligand-Target Pair
Affinity DataIC50: 9.00E+3nMAssay Description:Inhibition of human AChE using acetylthiocholine iodide as substrate treated 5 mins before substrate addition measured up to 4 mins by Ellman's metho...More data for this Ligand-Target Pair