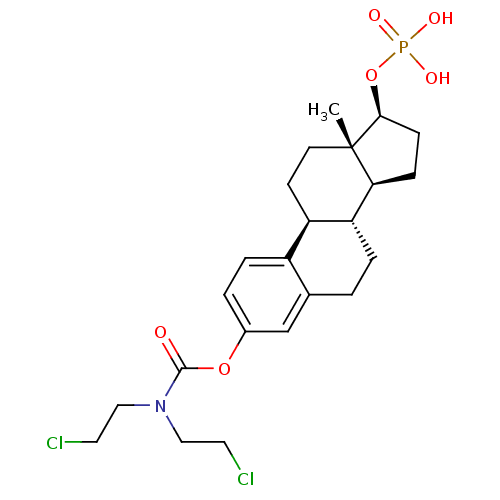
BDBM50333645 CHEMBL1756::Estramustine phosphate
SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OC(=O)N(CCCl)CCCl)ccc34)[C@@H]1CC[C@@H]2OP(O)(O)=O
InChI Key InChIKey=ADFOJJHRTBFFOF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50333645
Found 8 hits for monomerid = 50333645
TargetTyrosine-protein phosphatase non-receptor type 11(Human)
H. Lee Moffitt Cancer Center and Research Institute
Curated by ChEMBL
H. Lee Moffitt Cancer Center and Research Institute
Curated by ChEMBL
Affinity DataKd: 8.40E+3nMAssay Description:Binding affinity to recombinant SHP2 by surface plasmon responseMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Human)
H. Lee Moffitt Cancer Center and Research Institute
Curated by ChEMBL
H. Lee Moffitt Cancer Center and Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.71E+4nMAssay Description:Inhibition of SHP2 (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Human)
H. Lee Moffitt Cancer Center and Research Institute
Curated by ChEMBL
H. Lee Moffitt Cancer Center and Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.71E+4nMAssay Description:Inhibition of recombinant SHP2More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2(Human)
University of East Anglia
Curated by ChEMBL
University of East Anglia
Curated by ChEMBL
Affinity DataIC50: 3.55E+4nMAssay Description:Inhibition of 2-FAM-InsP5 binding to human SHIP2 catalytic domain (419 to 832 residues) assessed as change in polarization by fluorescence polarizati...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 6(Human)
H. Lee Moffitt Cancer Center and Research Institute
Curated by ChEMBL
H. Lee Moffitt Cancer Center and Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.04E+4nMAssay Description:Inhibition of recombinant SHP1More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2(Human)
University of East Anglia
Curated by ChEMBL
University of East Anglia
Curated by ChEMBL
Affinity DataIC50: 4.17E+4nMAssay Description:Inhibition of human SHIP2 catalytic domain (419 to 832 residues) phosphatase activity assessed as inhibition of Ins(1,3,4,5)P4 production using Ins(1...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Human)
H. Lee Moffitt Cancer Center and Research Institute
Curated by ChEMBL
H. Lee Moffitt Cancer Center and Research Institute
Curated by ChEMBL
Affinity DataIC50: 6.24E+4nMAssay Description:Inhibition of recombinant PTP1BMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 7(Human)
H. Lee Moffitt Cancer Center and Research Institute
Curated by ChEMBL
H. Lee Moffitt Cancer Center and Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.53E+5nMAssay Description:Inhibition of recombinant HePTPMore data for this Ligand-Target Pair