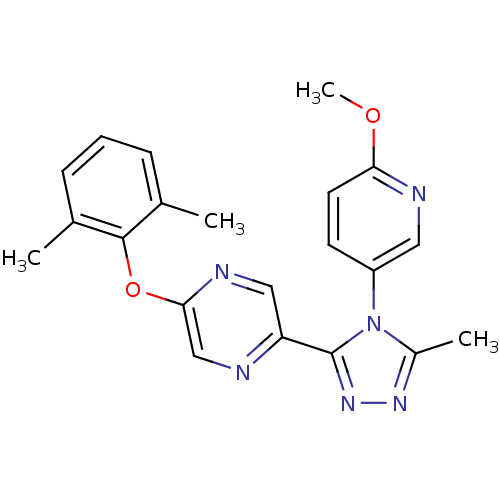
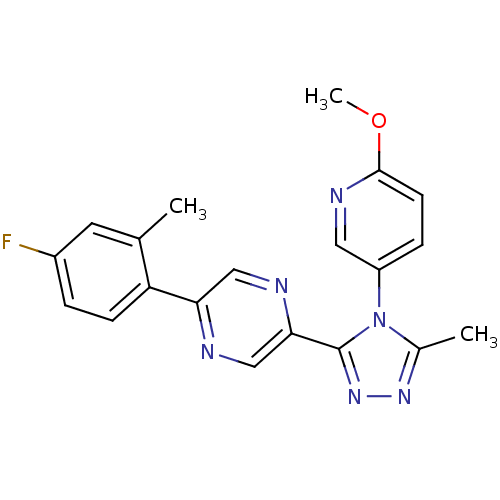
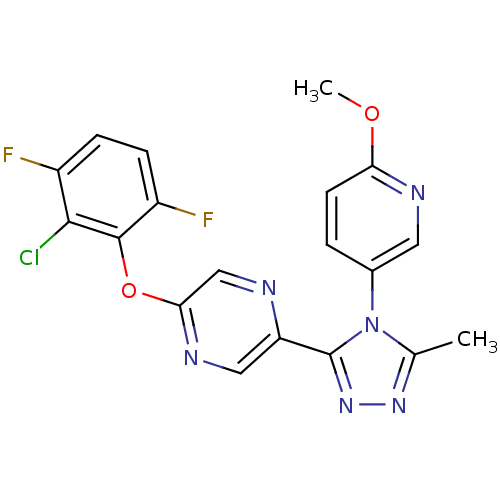
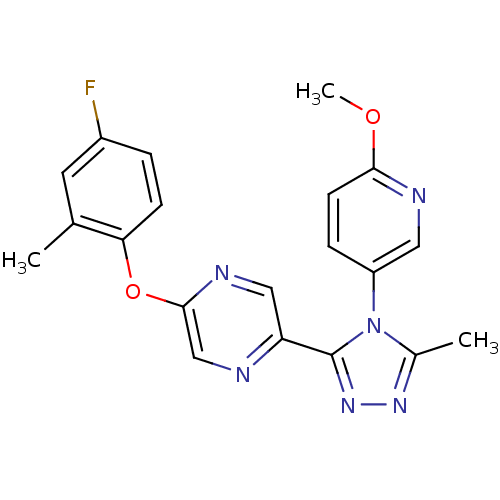
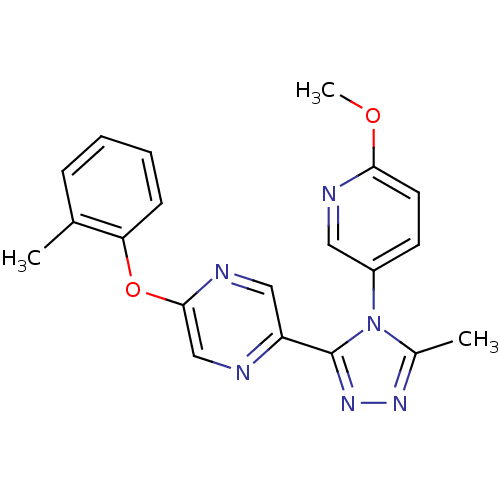
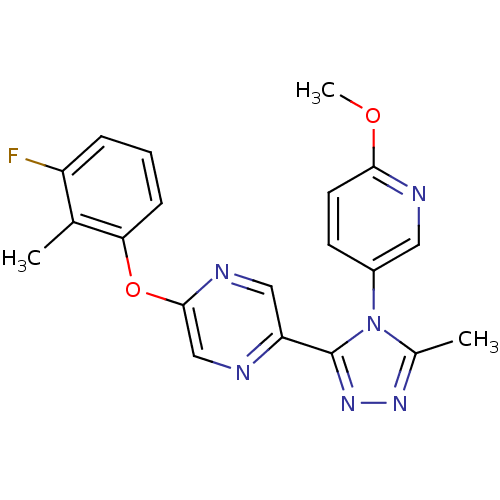
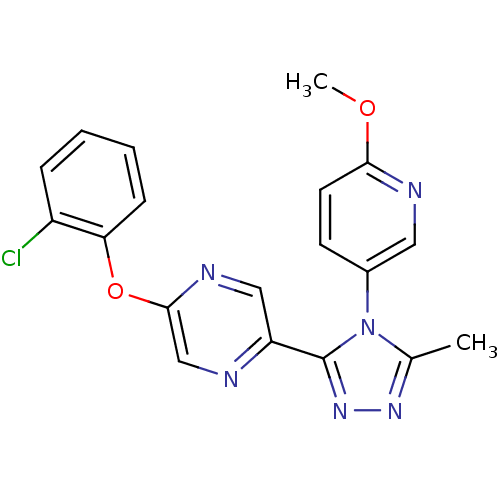
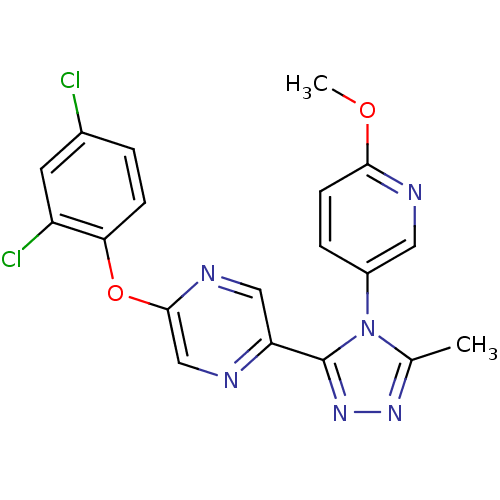
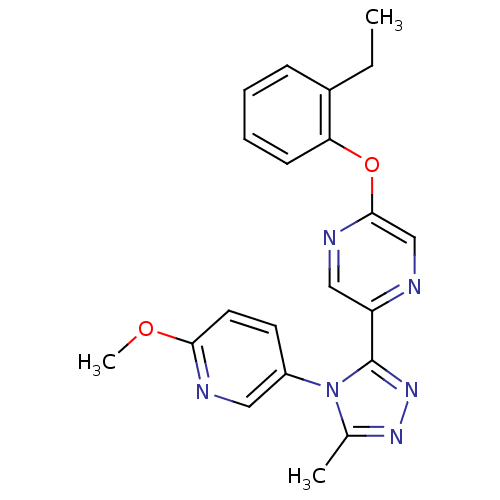
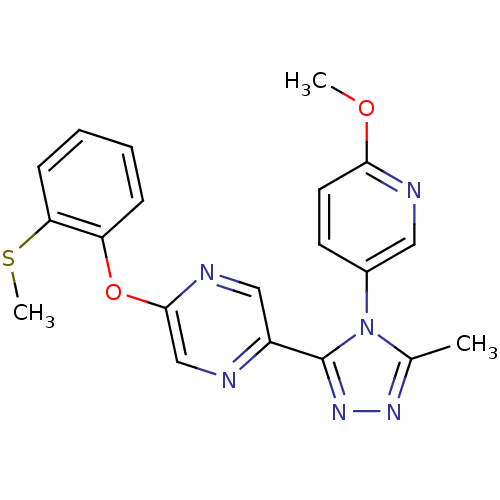
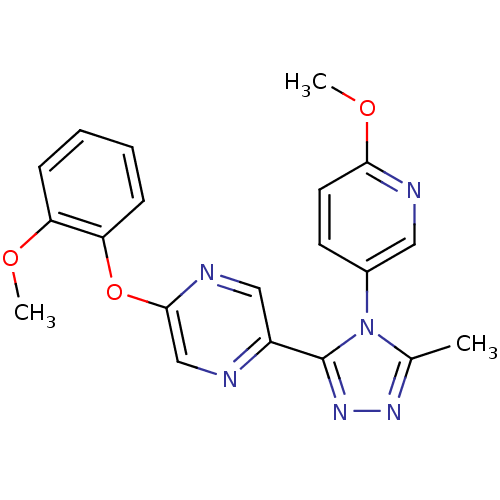
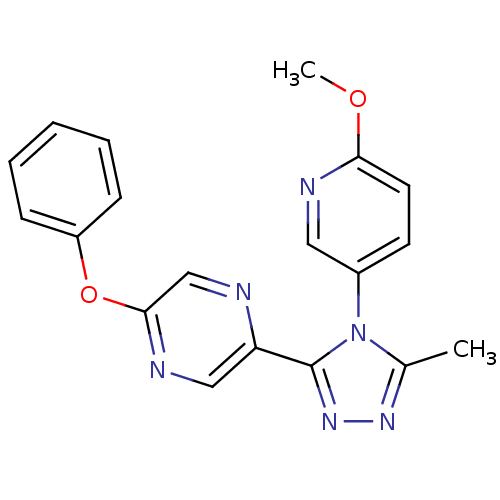
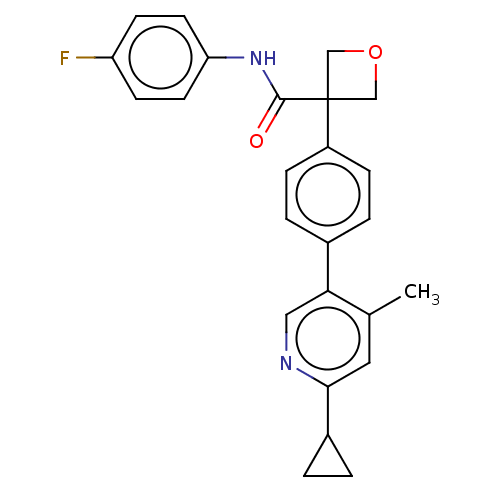
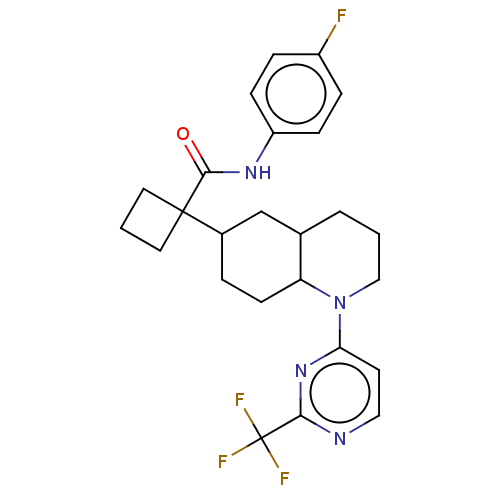
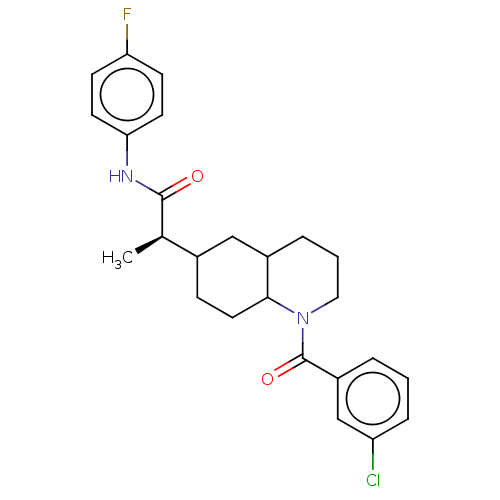
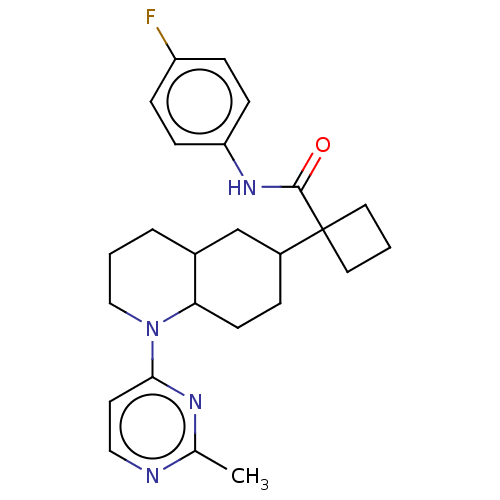
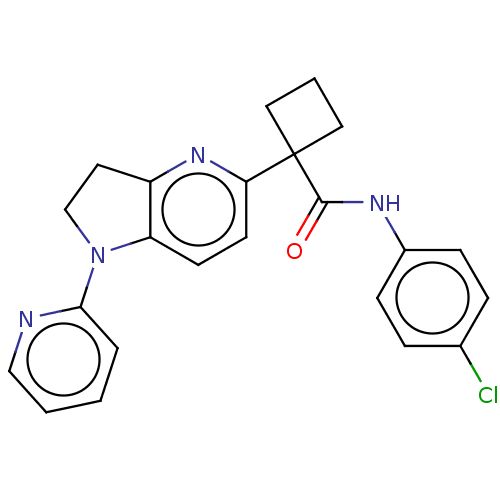
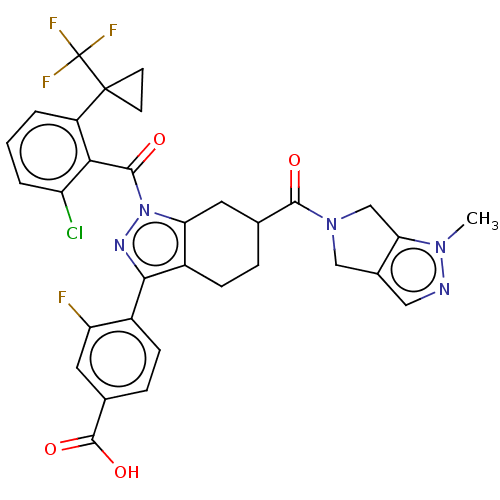
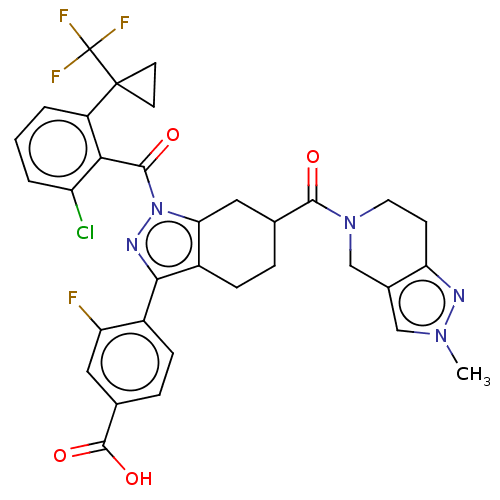
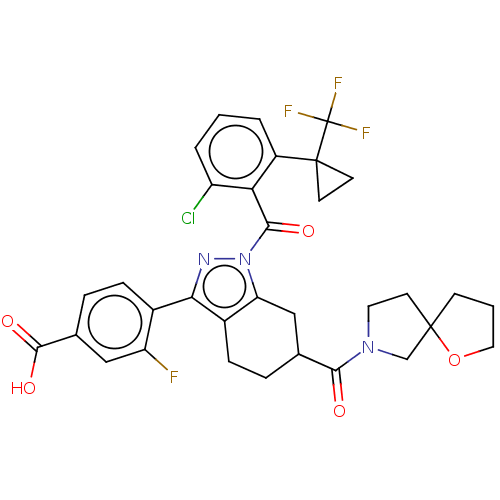
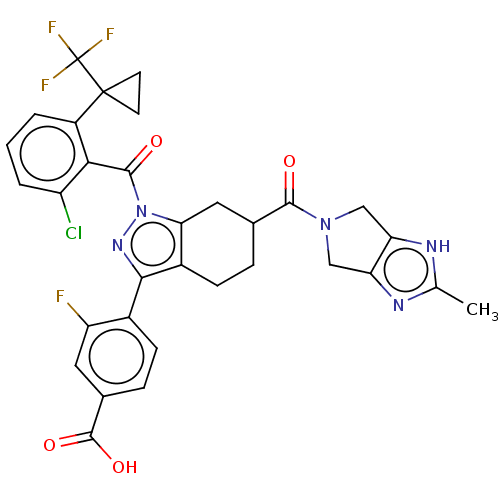
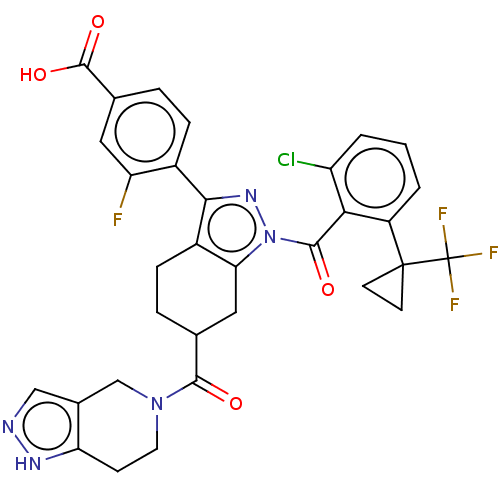
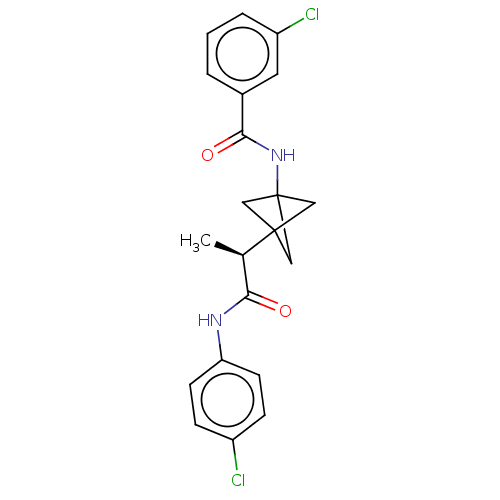
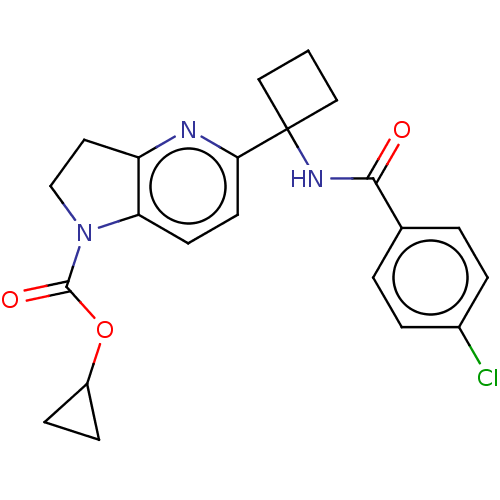
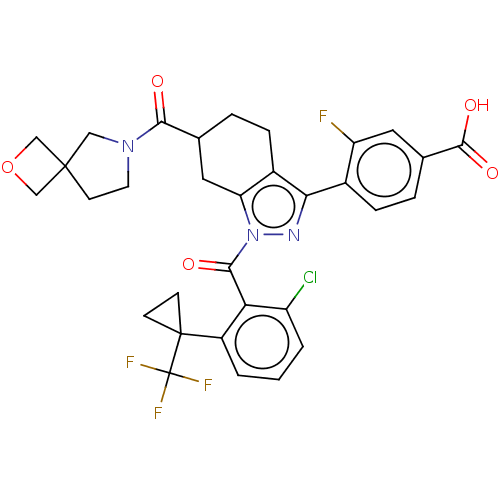
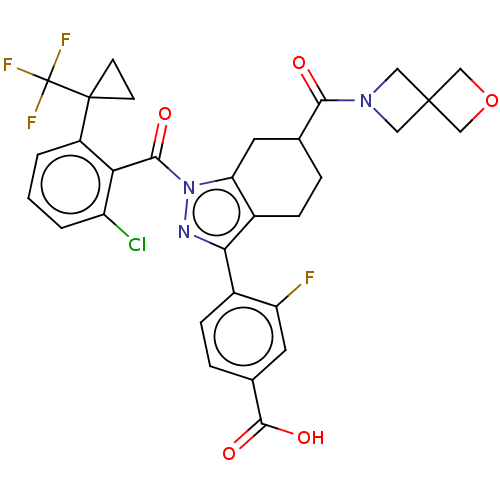
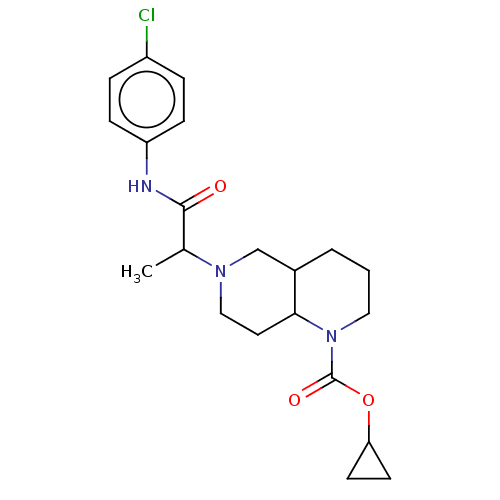
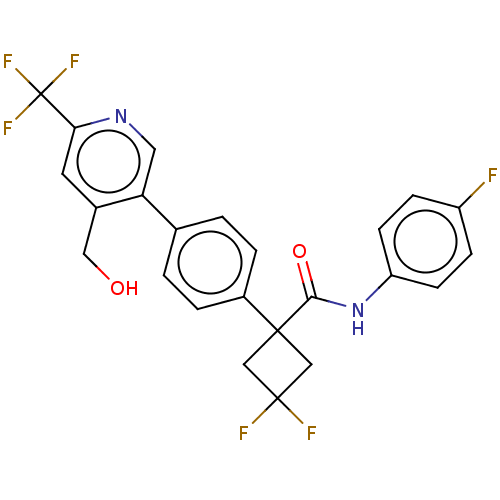
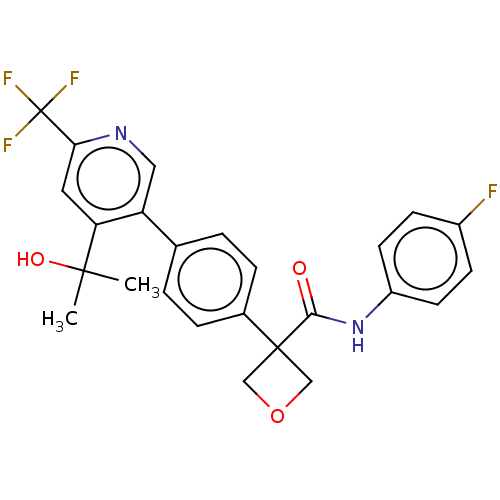
Affinity DataKi: 0.110nMAssay Description:Displacement of [3H] labeled-N-(4-Fluorophenyl)-3-(4-(4-(2-hydroxypropan-2-yl)-6-(trifluoromethyl)pyridin-3-yl)phenyl)oxetane-3-carboxamide from huma...More data for this Ligand-Target Pair
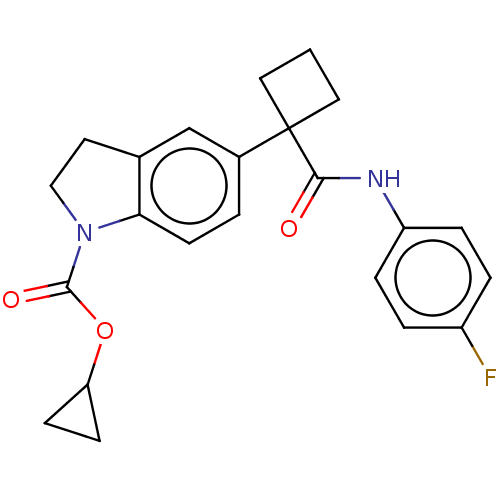
Affinity DataKi: 0.150nMAssay Description:Displacement of [3H] labeled-N-(4-Fluorophenyl)-3-(4-(4-(2-hydroxypropan-2-yl)-6-(trifluoromethyl)pyridin-3-yl)phenyl)oxetane-3-carboxamide from huma...More data for this Ligand-Target Pair
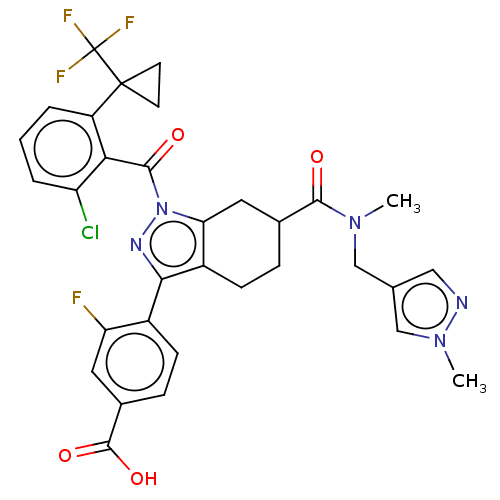
Affinity DataKi: 5.90nMAssay Description:Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assayMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assayMore data for this Ligand-Target Pair
Affinity DataKi: 8.80nMAssay Description:Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assayMore data for this Ligand-Target Pair
Affinity DataKi: 9.40nMAssay Description:Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assayMore data for this Ligand-Target Pair
Affinity DataKi: 12.5nMAssay Description:Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assayMore data for this Ligand-Target Pair
Affinity DataKi: 14.6nMAssay Description:Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assayMore data for this Ligand-Target Pair
Affinity DataKi: 23.6nMAssay Description:Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assayMore data for this Ligand-Target Pair
Affinity DataKi: 37.5nMAssay Description:Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assayMore data for this Ligand-Target Pair
Affinity DataKi: 52.3nMAssay Description:Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assayMore data for this Ligand-Target Pair
Affinity DataKi: 198nMAssay Description:Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assayMore data for this Ligand-Target Pair
Affinity DataKi: 253nMAssay Description:Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assayMore data for this Ligand-Target Pair
Affinity DataKi: 350nMAssay Description:Antagonist activity at human cloned oxytocin receptor by cell based beta-lactamase assayMore data for this Ligand-Target Pair
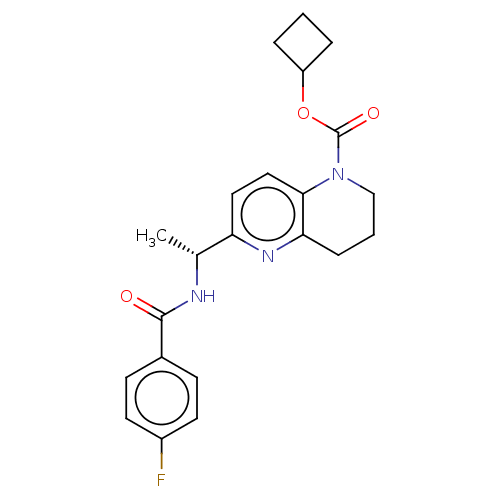
Affinity DataKi: 388nMAssay Description:Antagonist activity at human cloned vasopressin V1A receptor by cell based beta-lactamase assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.32E+3nMAssay Description:Antagonist activity at human cloned vasopressin V1A receptor by cell based beta-lactamase assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.32E+3nMAssay Description:Antagonist activity at human cloned vasopressin V1A receptor by cell based beta-lactamase assayMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Antagonist activity at human cloned vasopressin V2 receptor by cell based beta-lactamase assayMore data for this Ligand-Target Pair
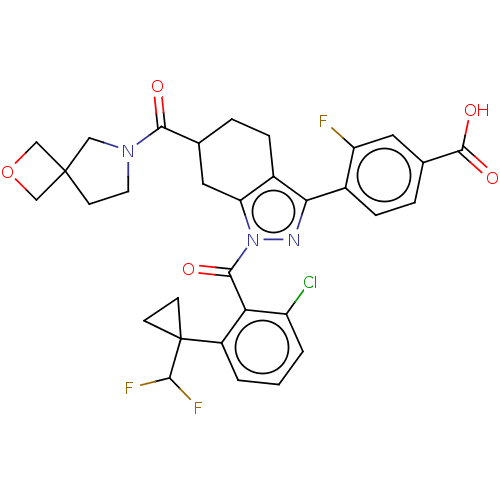
Affinity DataIC50: 0.0800nMAssay Description:Inhibition of IDO1 in IFNgamma/LPS stimulated human whole blood assessed as unbound concentration using kynurenine/tryptophan as substrate preincubat...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMAssay Description:Inhibition of IDO1 in IFNgamma/LPS stimulated human whole blood assessed as unbound concentration using kynurenine/tryptophan as substrate preincubat...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of IDO1 in human whole blood assessed as unbound concentrationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Inhibition of IDO1 in human whole blood assessed as unbound concentrationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.290nMAssay Description:Inhibition of IDO1 in IFNgamma/LPS stimulated human whole blood assessed as unbound concentration using kynurenine/tryptophan as substrate preincubat...More data for this Ligand-Target Pair
Affinity DataIC50: 0.290nMAssay Description:Inhibition of IDO1 in human whole blood assessed as unbound concentrationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.340nMAssay Description:Inhibition of human IDO1 assessed as unbound compound concentration by human whole blood assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.450nMAssay Description:Inhibition of human IDO1 assessed as unbound compound concentration by human whole blood assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of IDO1 in IFN-gamma stimulated human HeLa cells using L-tryptophan as substrate incubated for 48 hrs by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.630nMAssay Description:Inhibition of human IDO1 assessed as unbound compound concentration by human whole blood assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.730nMAssay Description:Inhibition of IDO1 in human whole blood assessed as unbound concentrationMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of IDO1 in IFN-gamma-stimulated human HeLa cells assessed as reduction in kynurenine production using L-Tryptophan as substrate incubated ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of IDO1 in IFNgamma/LPS stimulated human whole blood assessed as unbound concentration using kynurenine/tryptophan as substrate preincubat...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)