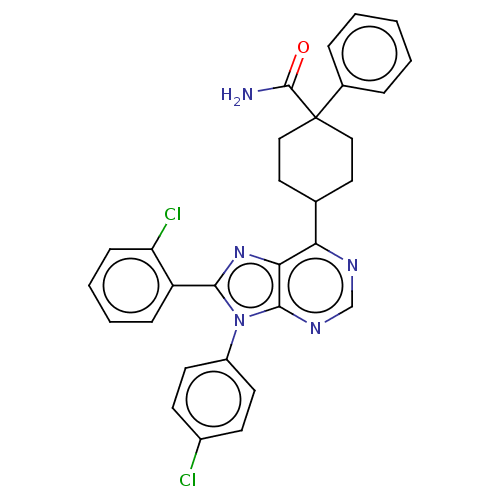
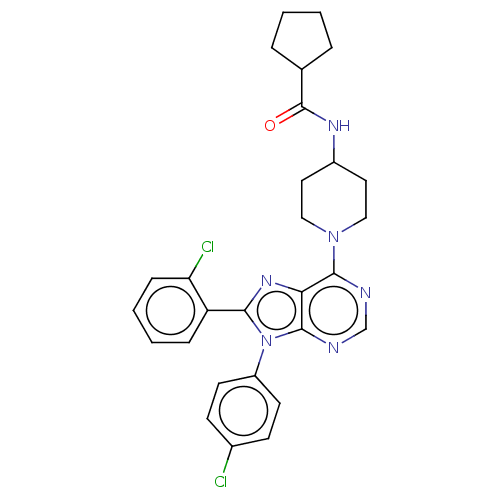
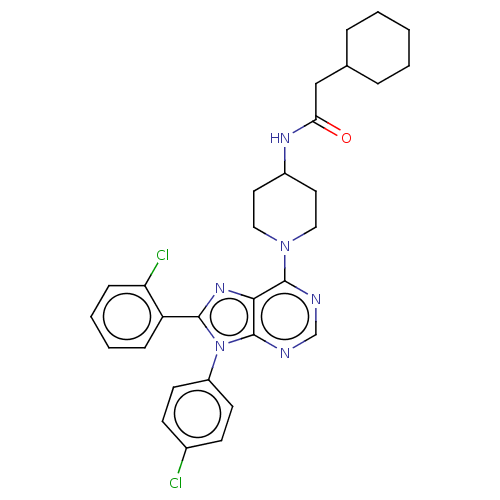
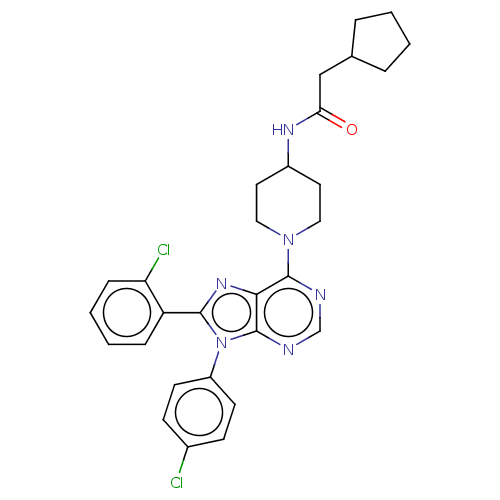
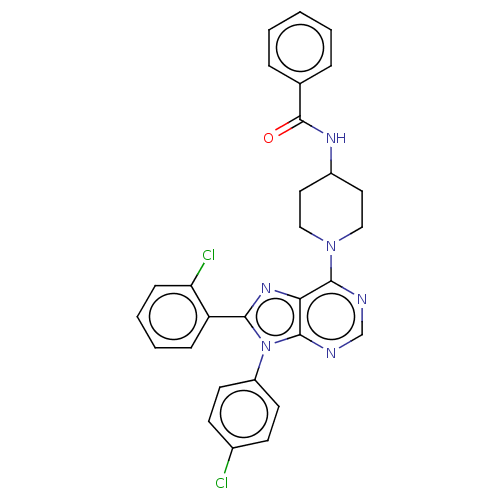
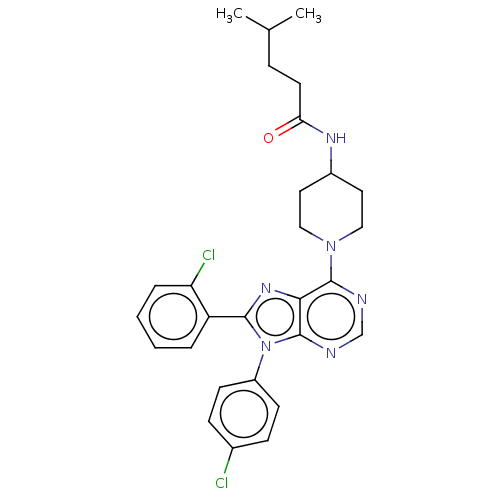
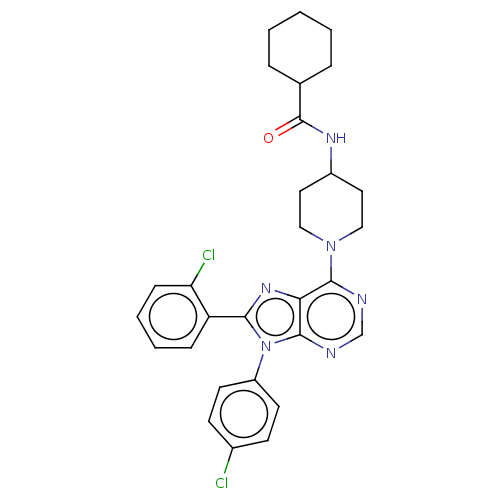
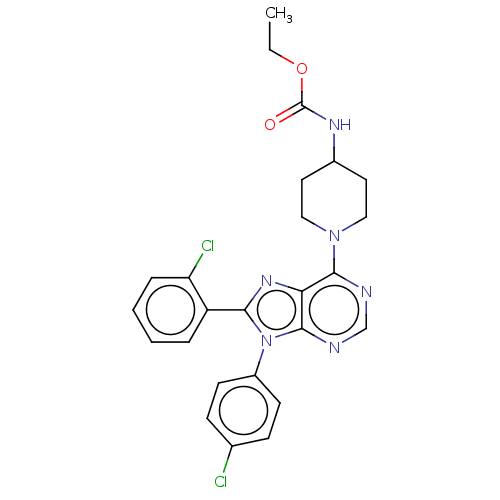
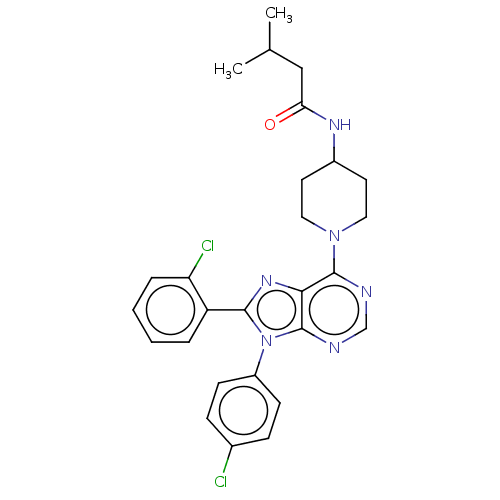
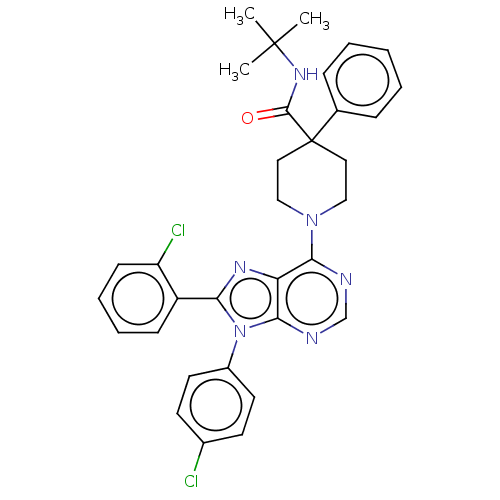
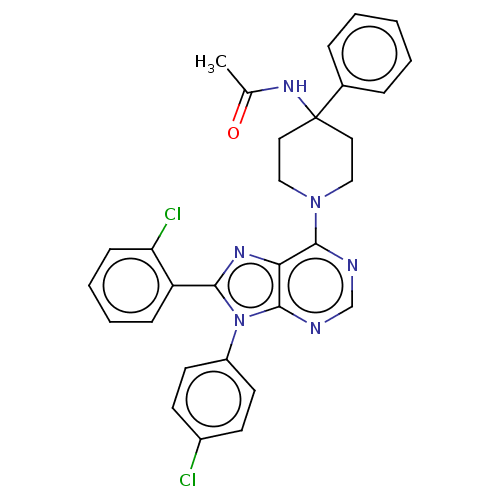
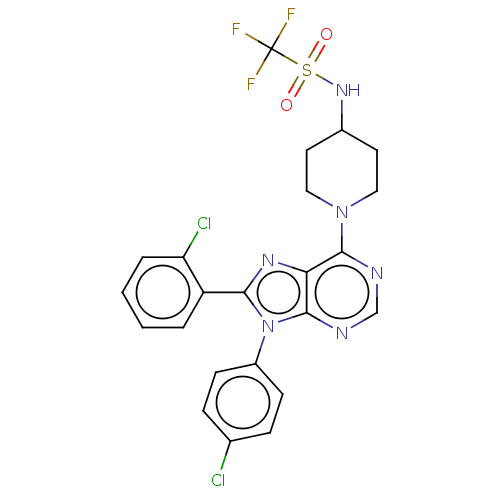
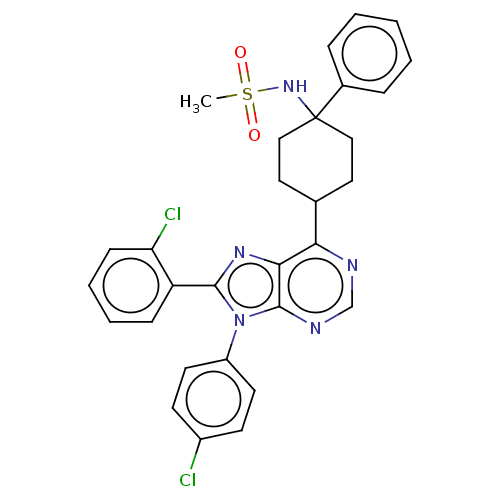
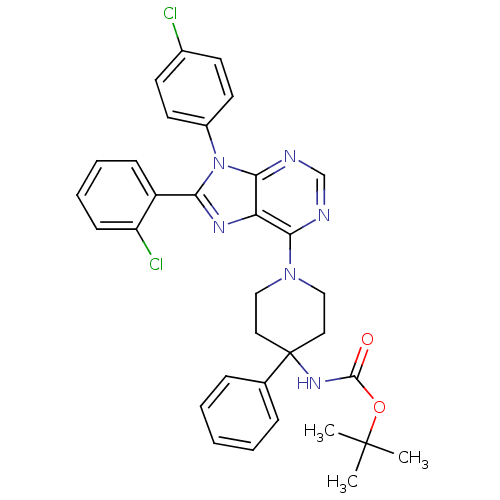
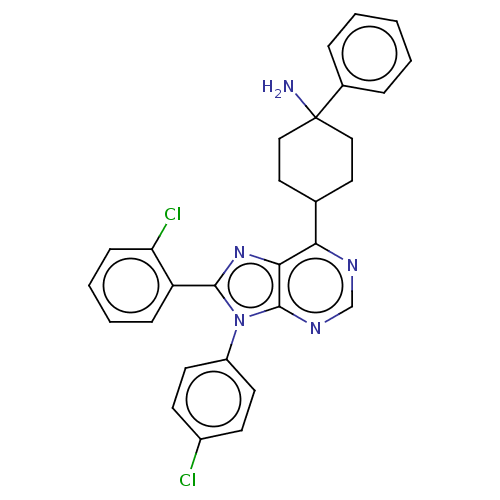
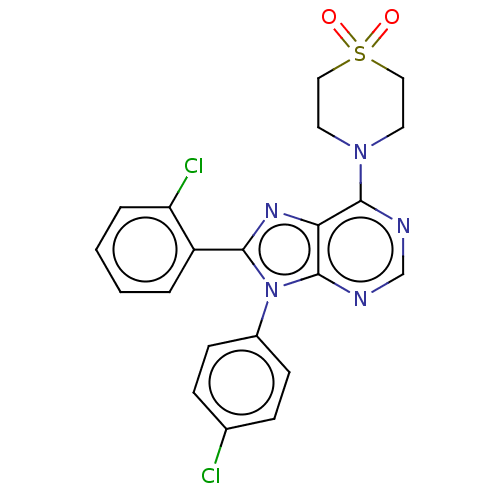
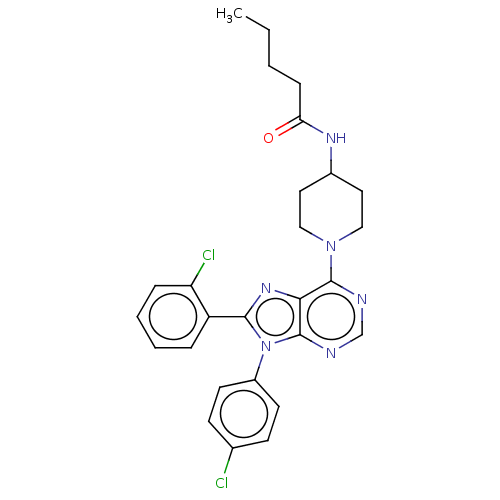
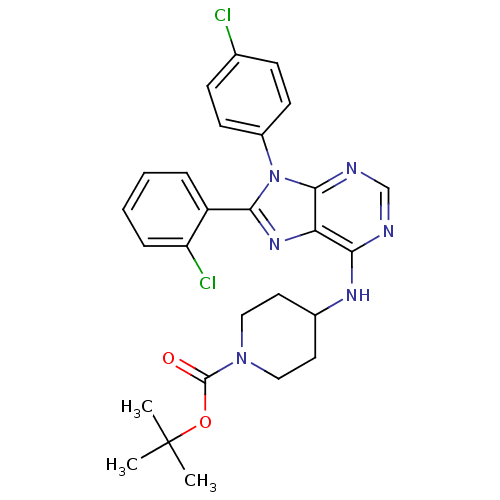
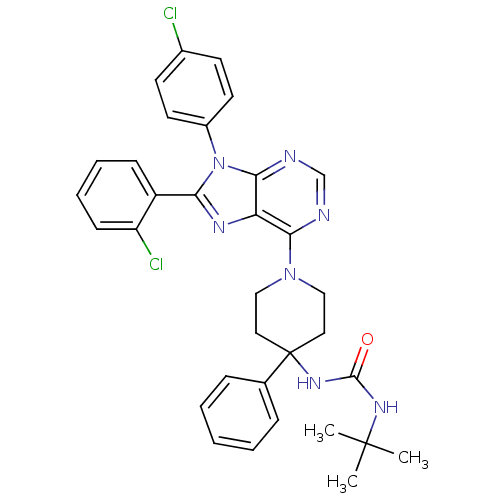
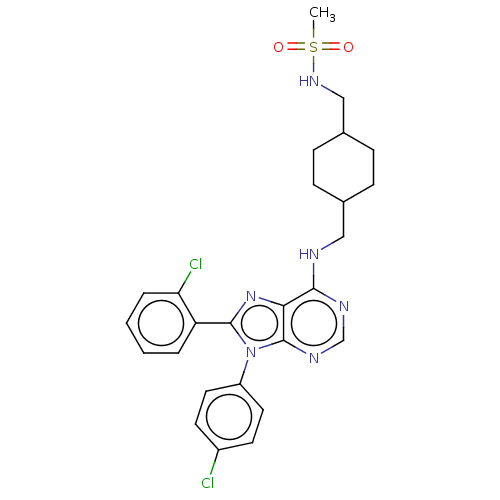
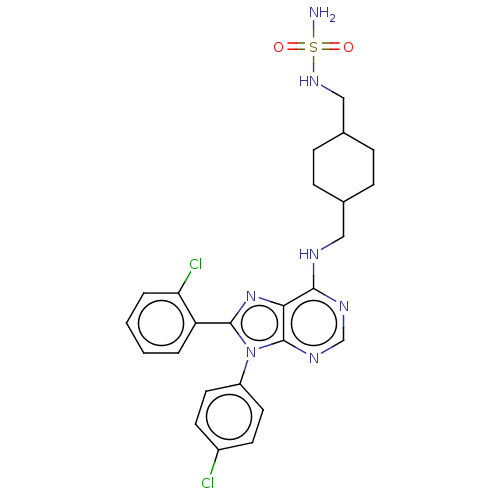
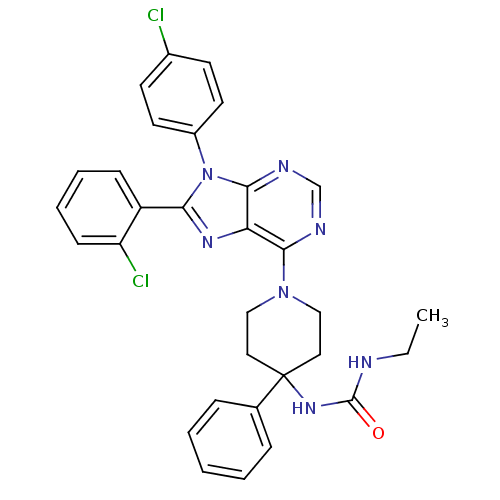
Affinity DataKi: 0.280nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 1.54nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 1.79nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 1.93nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 2.02nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 2.55nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 2.67nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 3.35nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 3.57nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 4.01nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 4.08nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 6.10nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 6.21nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 7.12nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 16.8nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 19.5nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 23.9nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 25.5nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 32.1nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 44.5nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 60.2nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 89.5nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 94.5nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 195nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 215nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 258nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 355nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 426nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 569nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 726nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 834nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 835nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 948nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 1.05E+3nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 1.66E+3nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 2.23E+3nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 2.36E+3nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 4.50E+3nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 4.65E+3nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 5.51E+3nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 1.06E+4nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 1.07E+4nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 1.36E+4nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 1.39E+4nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+4nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+4nMAssay Description:Further characterization of select compounds was performed using radioligand displacement of [3H]1 and equilibrium dissociation constant (Ki) values ...More data for this Ligand-Target Pair