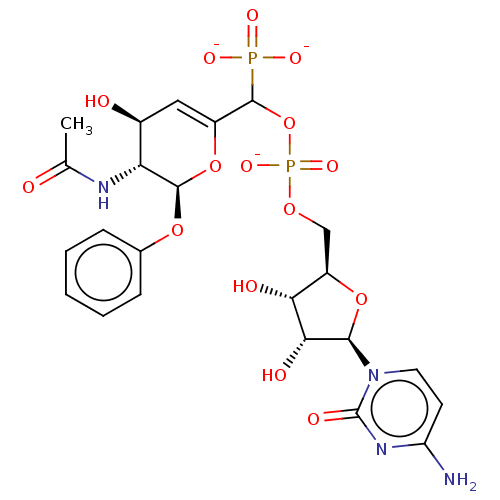
BDBM50501642 CHEMBL3629698
SMILES CC(=O)N[C@@H]1[C@@H](O)C=C(O[C@H]1Oc1ccccc1)C(OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(N)nc1=O)P([O-])([O-])=O
InChI Key InChIKey=QQLNDVODCNFGAI-UHFFFAOYSA-K
Data 2 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50501642
Found 2 hits for monomerid = 50501642
Affinity DataKi: 29nMAssay Description:Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC methodMore data for this Ligand-Target Pair
Affinity DataKi: 29nMAssay Description:Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC methodMore data for this Ligand-Target Pair