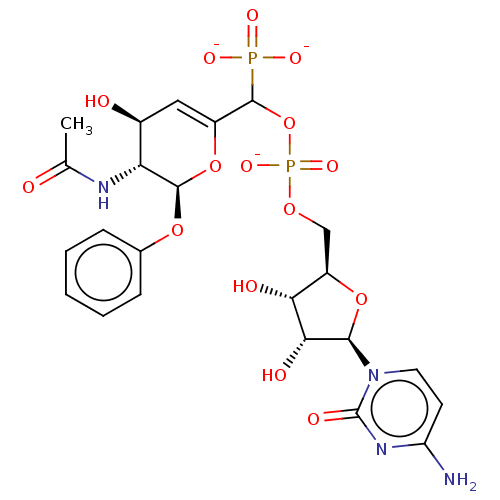
BDBM50501642 CHEMBL3629698
SMILES [Na;v0+].[Na;v0+].[Na;v0+].[#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@@H](-[#8])-[#6]=[#6](-[#8]-[#6@H]-1-[#8]-c1ccccc1)-[#6](-[#8]P([#8-])(=O)[#8]-[#6]-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8])-n1ccc(-[#7])nc1=O)P([#8-])([#8-])=O
InChI Key InChIKey=AUKRTEJKPBDVMA-WBLRXMGZSA-K
Data 2 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50501642
Found 2 hits for monomerid = 50501642
TargetBeta-galactoside alpha-2,6-sialyltransferase 1(Homo sapiens (Human))
Peking University
Curated by ChEMBL
Peking University
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC methodMore data for this Ligand-Target Pair
TargetBeta-galactoside alpha-2,6-sialyltransferase 1(Homo sapiens (Human))
Peking University
Curated by ChEMBL
Peking University
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC methodMore data for this Ligand-Target Pair