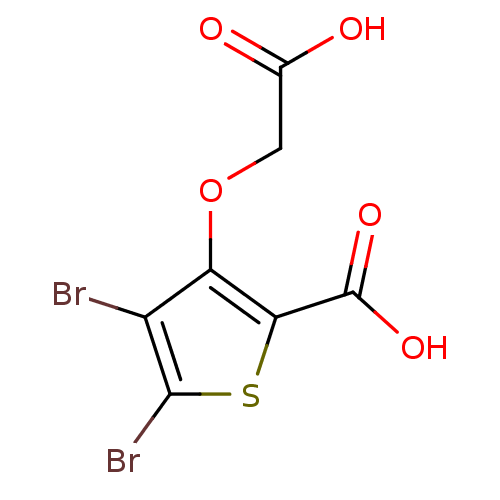
BDBM14244 4,5-dibromo-3-(carboxymethoxy)thiophene-2-carboxylic acid::CHEMBL373413::Monocyclic thiophene analog 10
SMILES OC(=O)COc1c(Br)c(Br)sc1C(O)=O
InChI Key InChIKey=JBNUJOXGKKSAFH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 14244
Found 3 hits for monomerid = 14244
Affinity DataKi: 1.80E+4nM ΔG°: -6.40kcal/molepH: 7.0 T: 2°CAssay Description:The enzymatic assay was carried out at room temperature in 96-well plates. The initial rate of PTPase-catalyzed hydrolysis of p-nitrophenol phosphate...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataKi: 1.80E+4nMAssay Description:Inhibition of PTP1B assessed as pNPP hydrolysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Wyeth Research
Curated by ChEMBL
Wyeth Research
Curated by ChEMBL
Affinity DataIC50: 2.90E+4nMAssay Description:Inhibition of PTP1B assessed as pNPP hydrolysisMore data for this Ligand-Target Pair