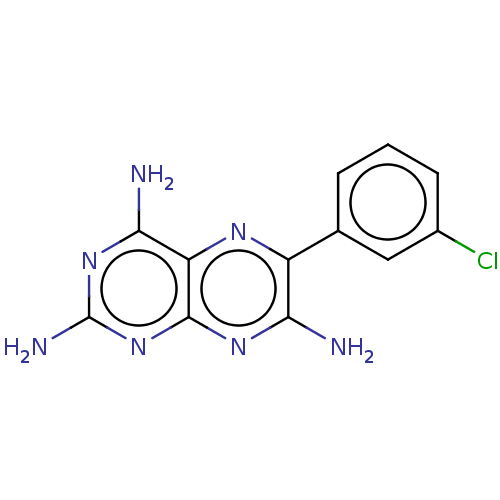
BDBM191572 6-(3-chlorophenyl)pteridine-2,4,7-triamine (15)::Epiblastin A
SMILES Nc1nc(N)c2nc(c(N)nc2n1)-c1cccc(Cl)c1
InChI Key InChIKey=ZWNKKZSRANLVEW-UHFFFAOYSA-N
Data 8 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 191572
Found 8 hits for monomerid = 191572
Affinity DataIC50: 500nMpH: 7.5Assay Description:A stock solution of 5 % DMSO (BioReagent for molecular biology, Sigma Aldrich) in water was prepared. The CK1 substrate peptide RRKDLHDDEEDEAMSITA (J...More data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMpH: 7.5Assay Description:A stock solution of 5 % DMSO (BioReagent for molecular biology, Sigma Aldrich) in water was prepared. The CK1 substrate peptide RRKDLHDDEEDEAMSITA (J...More data for this Ligand-Target Pair
Affinity DataIC50: 8.30E+3nMAssay Description:Single-point measurement analyses of Epiblastin A against a panel of 123 kinases was performed at Eurofins (former Milipore) using a radiometric-base...More data for this Ligand-Target Pair
Affinity DataIC50: 8.90E+3nMAssay Description:Single-point measurements analyses and subsequent dose-dependent investigations of selected pteridine derivatives for inhibition of CK1α were pe...More data for this Ligand-Target Pair
Affinity DataIC50: 2.42E+4nMAssay Description:Single-point measurement analyses of Epiblastin A against a panel of 123 kinases was performed at Eurofins (former Milipore) using a radiometric-base...More data for this Ligand-Target Pair
Affinity DataIC50: 2.74E+4nMAssay Description:Single-point measurement analyses of Epiblastin A against a panel of 123 kinases was performed at Eurofins (former Milipore) using a radiometric-base...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 2(Human)
Max Planck Institute of Molecular Physiology
Max Planck Institute of Molecular Physiology
Affinity DataIC50: 3.81E+4nMAssay Description:Single-point measurement analyses of Epiblastin A against a panel of 123 kinases was performed at Eurofins (former Milipore) using a radiometric-base...More data for this Ligand-Target Pair
TargetMAP kinase-interacting serine/threonine-protein kinase 2(Human)
Max Planck Institute of Molecular Physiology
Max Planck Institute of Molecular Physiology
Affinity DataIC50: 4.51E+4nMAssay Description:Single-point measurement analyses of Epiblastin A against a panel of 123 kinases was performed at Eurofins (former Milipore) using a radiometric-base...More data for this Ligand-Target Pair