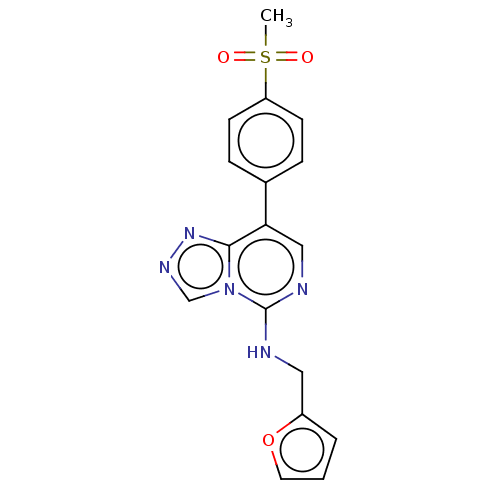
BDBM225230 EED226::US11013745, Compound EED226
SMILES CS(=O)(=O)c1ccc(cc1)-c1cnc(NCc2ccco2)n2cnnc12
InChI Key InChIKey=DYIRSNMPIZZNBK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 93 hits for monomerid = 225230
Found 93 hits for monomerid = 225230
Affinity DataIC50: 12nMAssay Description:Inhibition of biotinylated H3K27Me3 peptide (19 to 33 residues) binding to His-tagged EED (1 to 441 residues) (unknown origin) preincubated for 15 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibition of EED ( 1 to 441 residues) (unknown origin) pre-incubated for 15 mins before biotin-H3K27me3 (19 to 33 residues) addition and further inc...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of EED (unknown origin)More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Human)
Novartis Institutes For Biomedical Research
Novartis Institutes For Biomedical Research
Affinity DataIC50: 20nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Binding affinity to EED in PRC2 complex (unknown origin) using H3K27me0 (21 to 44 residue) peptide as substrate by LC-MS analysisMore data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Human)
Novartis Institutes For Biomedical Research
Novartis Institutes For Biomedical Research
Affinity DataIC50: 23.4nMpH: 8.0Assay Description:To assess the compounds potency in the H3K27me0 peptide (21–44)-based PRC2 enzymatic assay, compounds were serially diluted three-fold in DMSO t...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of EED (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of full length N-terminal His-6x tagged EED (1 to 441 residues) (unknown origin) incubated for 1 hr by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inhibition of His tagged EED (1 to 441) (unknown origin) using biotin-H3K27me3 peptide as substrate by AlphaScreen competition binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Displacement of UNC5114-biotin tracer ligand from N-terminal 6His-tagged EED (1 to 441 residues) (unknown origin) expressed in Escherichia coli Roset...More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH1/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Human)
Novartis Institutes For Biomedical Research
Novartis Institutes For Biomedical Research
Affinity DataIC50: 50nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED/Polycomb protein SUZ12/Zinc finger protein AEBP2(Human)
Novartis Institutes For Biomedical Research
Novartis Institutes For Biomedical Research
Affinity DataIC50: 53.5nMpH: 8.0Assay Description:To assess the compounds potency in the H3K27me0 peptide (21–44)-based PRC2 enzymatic assay, compounds were serially diluted three-fold in DMSO t...More data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor alpha/beta(Mouse)
University of Jinany
Curated by ChEMBL
University of Jinany
Curated by ChEMBL
Affinity DataKd: 82nMAssay Description:Binding affinity to PRC2 complex of EZH2-EED-SUZ12 (unknown origin) assessed as dissociation constant by isothermal titration calorimetry methodMore data for this Ligand-Target Pair
Affinity DataKd: 82nMpH: 8.0 T: 2°CAssay Description:ITC experiments were performed by Auto ITC200 (Microcal) at 25 °C. ITC sample cell was filled with 10 μM PRC2 in titration buffer (25 mM HEPES p...More data for this Ligand-Target Pair
Affinity DataKd: 82nMAssay Description:Binding affinity to EED (unknown origin) by ITC methodMore data for this Ligand-Target Pair
TargetHistone-binding protein RBBP4/Histone-lysine N-methyltransferase EZH2/Polycomb protein EED [40-441]/Polycomb protein SUZ12(Human)
Shanghai Institute of Material Medica, Chinese Academy of Sciences
US Patent
Shanghai Institute of Material Medica, Chinese Academy of Sciences
US Patent
Affinity DataIC50: 104nMAssay Description:The TR-FRET method was used to measure the PRC2 enzyme activity. First, the enzyme was mixed with compounds at different concentrations and incubated...More data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2/Polycomb protein EED [40-441]/Polycomb protein SUZ12 [559-739](Human)
Novartis Institutes For Biomedical Research
Novartis Institutes For Biomedical Research
Affinity DataKd: 114nMpH: 8.0 T: 2°CAssay Description:ITC experiments were performed by Auto ITC200 (Microcal) at 25 °C. ITC sample cell was filled with 10 μM PRC2 in titration buffer (25 mM HEPES p...More data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Inhibition of EED in human G401 cells assessed as reduction in global H3K27me3 level after 48 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Inhibition of EED in human G401 cells assessed as reduction in global H3K27me3 level after 48 hrs by ELISAMore data for this Ligand-Target Pair
TargetFibroblast growth factor receptor 2 [406-821](Human)
Novartis Institutes For Biomedical Research
Novartis Institutes For Biomedical Research
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
TargetFibroblast growth factor receptor 4 [388-802](Human)
Novartis Institutes For Biomedical Research
Novartis Institutes For Biomedical Research
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase 8 [30-404](Human)
Novartis Institutes For Biomedical Research
Novartis Institutes For Biomedical Research
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2 [807-1356](Human)
Novartis Institutes For Biomedical Research
Novartis Institutes For Biomedical Research
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
TargetInterleukin-1 receptor-associated kinase 1 [184-712](Human)
Novartis Institutes For Biomedical Research
Novartis Institutes For Biomedical Research
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3 [563-993,D835Y](Human)
Novartis Institutes For Biomedical Research
Novartis Institutes For Biomedical Research
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase kinase kinase kinase 4(Human)
Novartis Institutes For Biomedical Research
Novartis Institutes For Biomedical Research
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor alpha [551-1089,V561D](Human)
Novartis Institutes For Biomedical Research
Novartis Institutes For Biomedical Research
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
TargetAlpha-2C adrenergic receptor(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of alpha2c adrenergic receptor (unknown origin)More data for this Ligand-Target Pair
TargetAlpha-2A adrenergic receptor(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.50E+4nMAssay Description:Antagonist activity against alpha2a adrenergic receptor (unknown origin)More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human Phosphodiesterase 4DMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG by binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-1(Human)
Novartis Institutes For Biomedical Research
Novartis Institutes For Biomedical Research
Affinity DataIC50: 3.00E+4nMAssay Description:All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383].More data for this Ligand-Target Pair