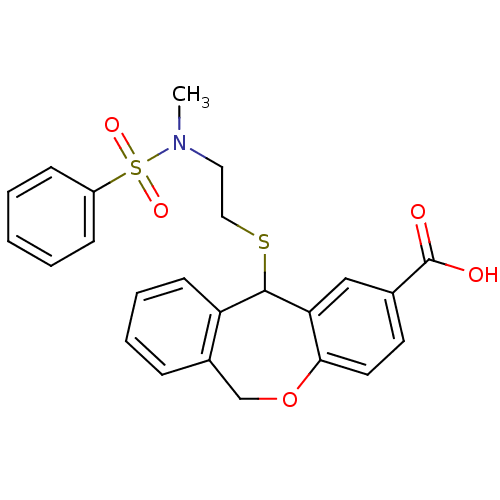
BDBM50002755 11-[2-(Benzenesulfonyl-methyl-amino)-ethylsulfanyl]-6,11-dihydro-dibenzo[b,e]oxepine-2-carboxylic acid::CHEMBL114424
SMILES CN(CCSC1c2ccccc2COc2ccc(cc12)C(O)=O)S(=O)(=O)c1ccccc1
InChI Key InChIKey=GODBHXSVNOESLI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50002755
Found 4 hits for monomerid = 50002755
Affinity DataKi: 33nMAssay Description:Binding affinity at TXA2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig plateletsMore data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Inhibition of the thromboxane A2 receptor assayed by binding to guinea pig platelets using [3H]-U-46,619 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Compound was tested for its inhibitory activity against TXA2 synthase obtained from bovine platelet microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibitory effect of the compound against thromboxane A2 synthase binding to bovine plateletsMore data for this Ligand-Target Pair