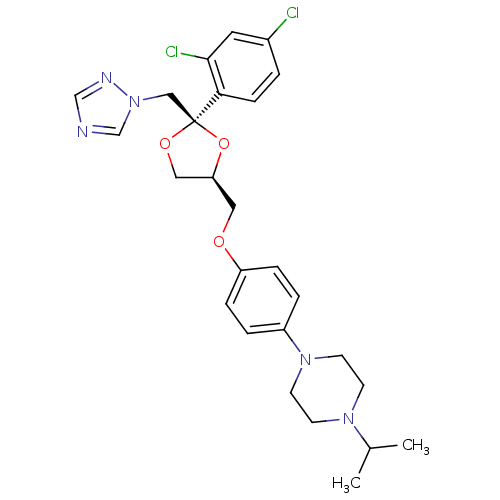
BDBM50375318 Fungistat::Gyno-Terazol::Panlomyc::R-42470::TERCONAZOLE::Terazol::Terazol 3::Terazol 7::med.21724, Compound Terconazole
SMILES CC(C)N1CCN(CC1)c1ccc(OC[C@H]2CO[C@@](Cn3cncn3)(O2)c2ccc(Cl)cc2Cl)cc1
InChI Key InChIKey=BLSQLHNBWJLIBQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50375318
Found 8 hits for monomerid = 50375318
Affinity DataIC50: 410nMAssay Description:Inhibition of rat spleen microsome HO-1 preincubated for 10 mins followed by beta-NADPH addition and measured after 15 mins by gas chromatographyMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human CYP51 expressed in Topp 3 cells by lanosterol demethylase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of rat brain microsome HO-2 preincubated for 10 mins followed by beta-NADPH addition and measured after 15 mins by gas chromatographyMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
Affinity DataEC50: 8.90E+3nMAssay Description:Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysisMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Rat)
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
Affinity DataEC50: 9.10E+3nMAssay Description:Activation of rat PXR expressed in human HepG2 cells after 24 hrs by luciferase reporter gene based luminescent analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 1.22E+4nMAssay Description:This is a review article.More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
Affinity DataEC50: 1.41E+4nMAssay Description:Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 1.53E+4nMAssay Description:This is a review article.More data for this Ligand-Target Pair