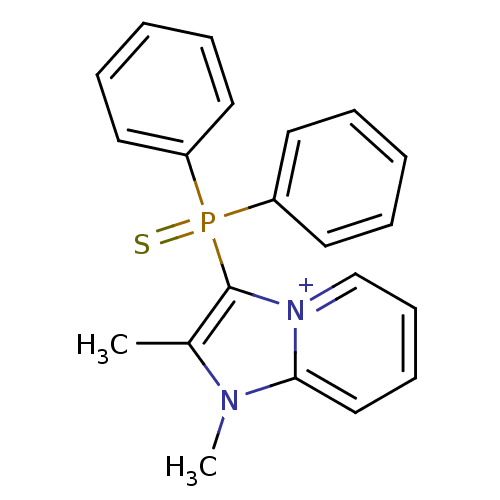
BDBM89034 (1,2-dimethyl-3-imidazo[1,2-a]pyridin-4-iumyl)-diphenyl-sulfanylidenephosphorane;methyl sulfate::(1,2-dimethylimidazo[1,2-a]pyridin-4-ium-3-yl)-diphenyl-sulfanylidene--phosphane;methyl sulfate::(1,2-dimethylimidazo[1,2-a]pyridin-4-ium-3-yl)-diphenyl-thioxo-phosphorane;methyl sulfate::CHEMBL1598051::MLS002703110::SMR001566915::cid_56643192
SMILES Cc1c([n+]2ccccc2n1C)P(=S)(c1ccccc1)c1ccccc1
InChI Key InChIKey=DHDHZKHTNUDMEA-UHFFFAOYSA-N
Data 8 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 89034
Found 8 hits for monomerid = 89034
TargetProthrombin(Bos taurus (Bovine))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 6.28E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford Burnham Medical Research Institute (SBMRI, La Jolla, CA...More data for this Ligand-Target Pair
Target26S proteasome non-ATPase regulatory subunit 14(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 5.42E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford Burnham Medical Research Institute (SBMRI, La Jolla, CA...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as inhibition of NPS-induced ERK activation after 20 ...More data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Antagonist activity at neuropeptide S receptor (unknown origin) assessed as intracellular calcium level by cell based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as cAMP level after 30 mins by phosphate-buffered sal...More data for this Ligand-Target Pair
Affinity DataIC50: 84nMAssay Description:Displacement of [125I]Tyr10-NPS from neuropeptide S receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Antagonist activity at neuropeptide S receptor (unknown origin) assessed as cAMP level by cell based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Antagonist activity at neuropeptide S receptor (unknown origin) expressed in CHO cells assessed as inhibition of NPS-induced calcium mobilization aft...More data for this Ligand-Target Pair