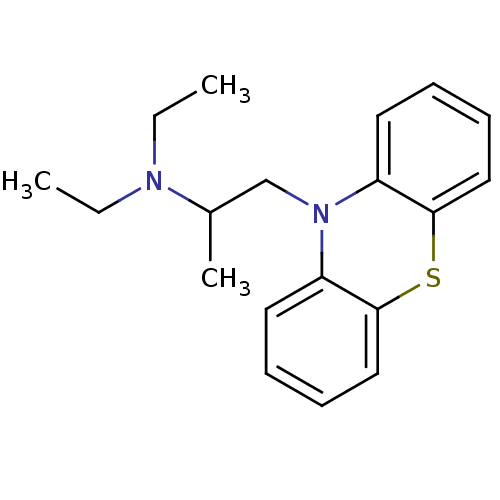
BDBM8958 10-(2-diethylaminopropyl)phenothiazine::CHEMBL1200970::CHEMBL1206::Ethopropazine::diethyl[1-(10H-phenothiazin-10-yl)propan-2-yl]amine
SMILES CCN(CC)C(C)CN1c2ccccc2Sc2ccccc12
InChI Key InChIKey=CDOZDBSBBXSXLB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 8958
Found 5 hits for monomerid = 8958
Affinity DataIC50: 300nMAssay Description:Inhibition of human plasma BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of human erythrocytes BChEMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+5nMAssay Description:Inhibition of human plasma AChEMore data for this Ligand-Target Pair
Affinity DataIC50: 720nMAssay Description:Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assayMore data for this Ligand-Target Pair
Affinity DataIC50: 260nMAssay Description:Inhibition of human erythrocytes AChEMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)