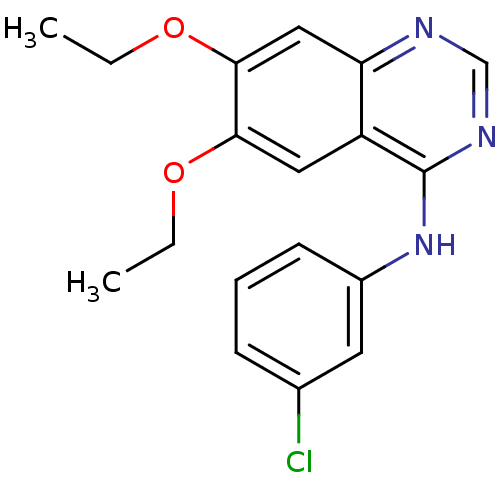
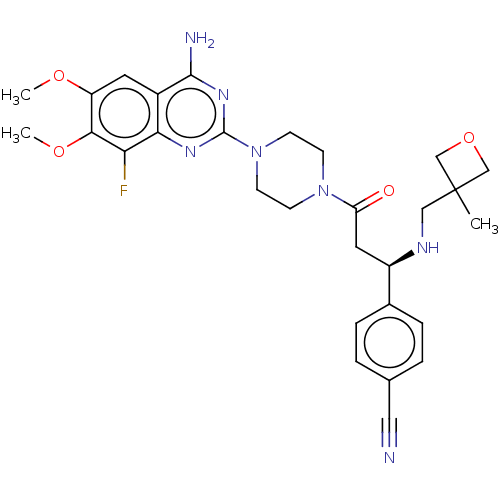
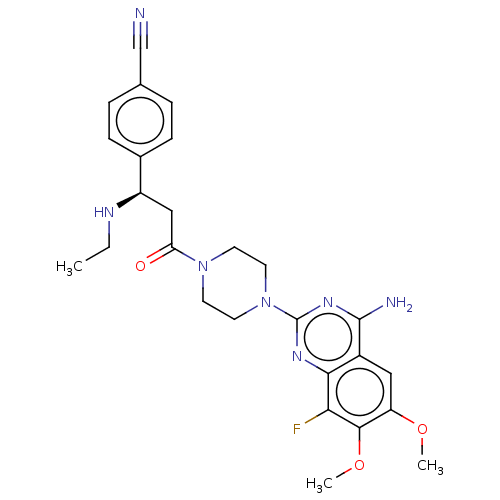
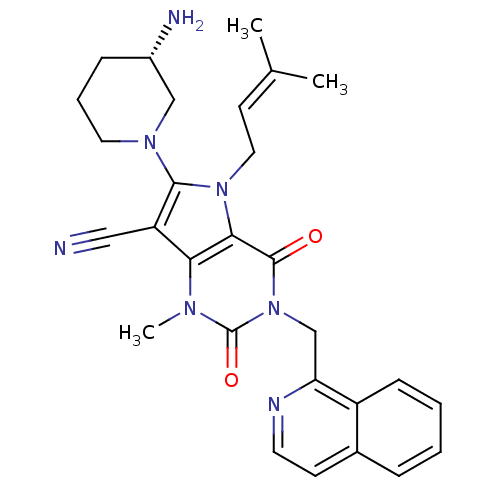
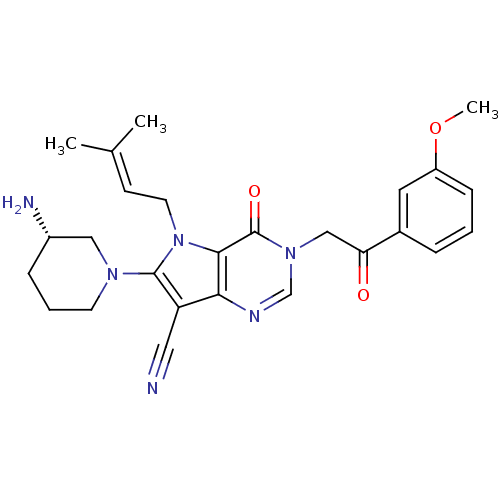
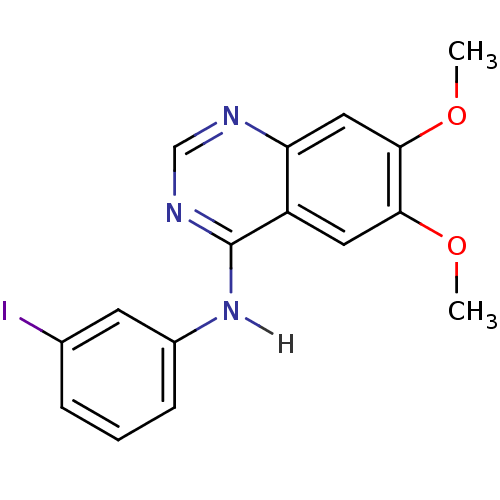
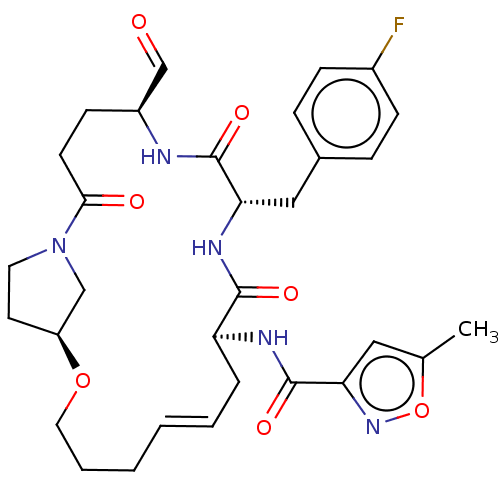
Affinity DataKi: 20nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
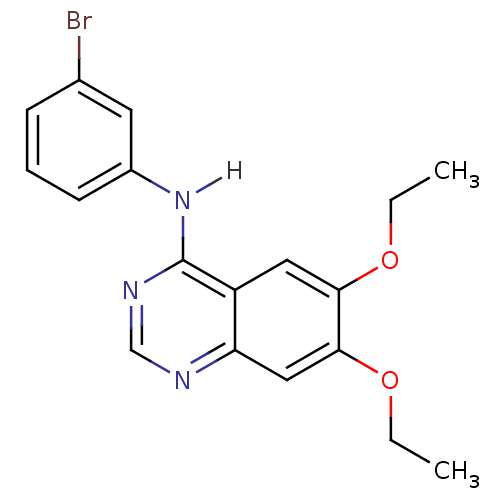
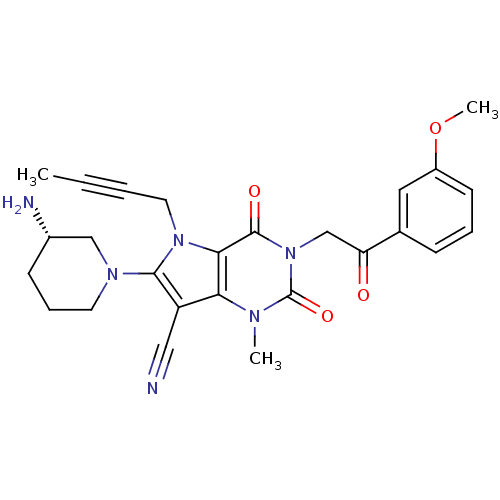
Affinity DataKi: 80nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
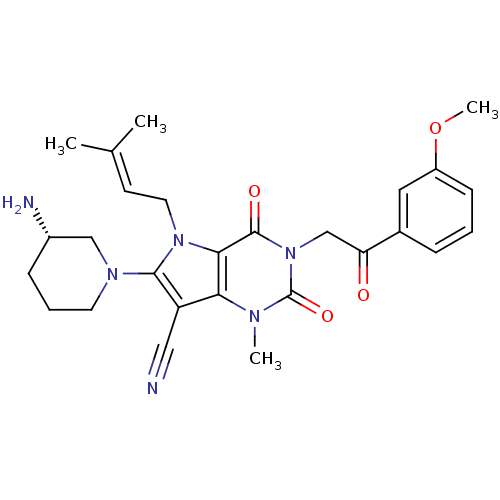
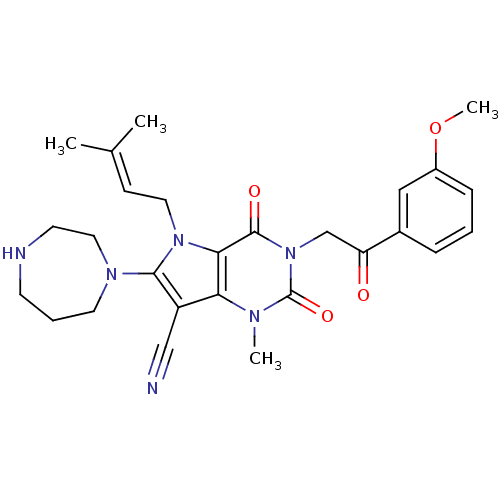
Affinity DataKi: 710nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
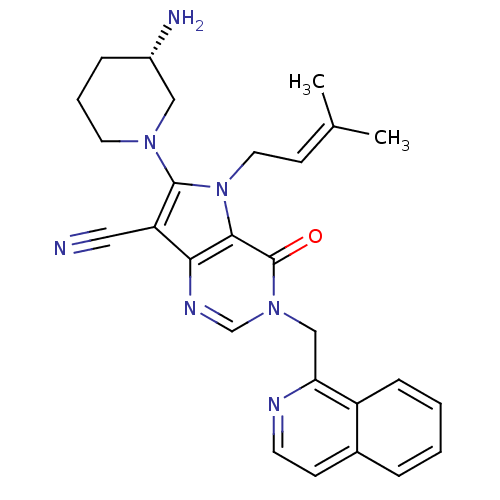
Affinity DataKi: 1.35E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.44E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetLeucine--tRNA ligase, cytoplasmic(Saccharomyces cerevisiae S288c)
Anacor Pharmaceuticals
Curated by ChEMBL
Anacor Pharmaceuticals
Curated by ChEMBL
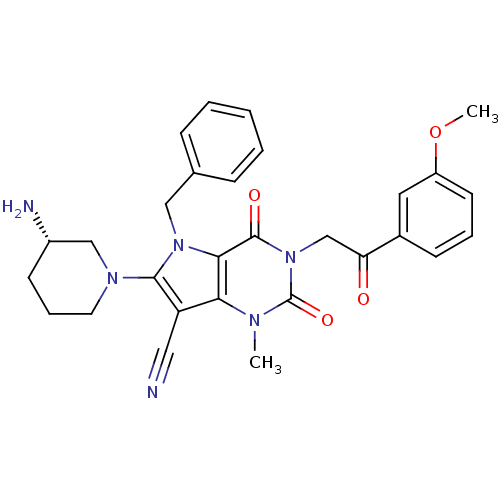
Affinity DataKi: 1.85E+3nMAssay Description:Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase after 20 minsMore data for this Ligand-Target Pair
Affinity DataKi: 2.27E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.75E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.33E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.73E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.55E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 6.00E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 7.28E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 7.72E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 8.40E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 8.73E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 9.09E+3nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.33E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.98E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.85E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetLeucine--tRNA ligase, cytoplasmic(Saccharomyces cerevisiae S288c)
Anacor Pharmaceuticals
Curated by ChEMBL
Anacor Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 3.14E+4nMAssay Description:Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase after 2 minsMore data for this Ligand-Target Pair
Affinity DataKi: 4.06E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.66E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.80E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 6.32E+4nMAssay Description:Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus systemMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Lawrence Berkeley National Laboratory
Curated by ChEMBL
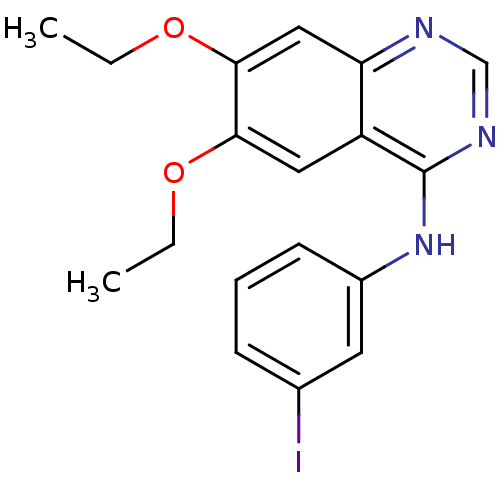
Affinity DataIC50: 0.380nMAssay Description:Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranesMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Lawrence Berkeley National Laboratory
Curated by ChEMBL
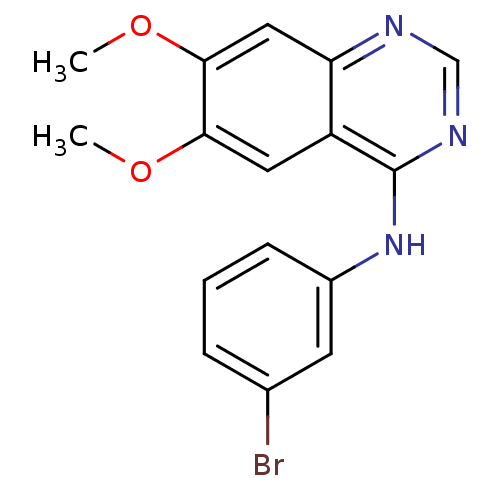
Affinity DataIC50: 0.410nMAssay Description:Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus systemMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus systemMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Affinity DataIC50: 0.640nMAssay Description:Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranesMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Affinity DataIC50: 0.660nMAssay Description:Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranesMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus systemMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus systemMore data for this Ligand-Target Pair
TargetCobra venom factor/Complement factor B/Complement factor D(Naja kaouthia (Monocled cobra) (Naja siamensis))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:CVF-Bb complex prepared from purified cobra venom factor (1 μM), recombinant human complement factor B (expressed in drosophila cells and purifi...More data for this Ligand-Target Pair
TargetCobra venom factor/Complement factor B/Complement factor D(Naja kaouthia (Monocled cobra) (Naja siamensis))
Novartis
US Patent
Novartis
US Patent
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:CVF-Bb complex prepared from purified cobra venom factor (1 μM), recombinant human complement factor B (expressed in drosophila cells and purifi...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus systemMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus systemMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Affinity DataIC50: 1.05nMAssay Description:Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranesMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cellsMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Affinity DataIC50: 1.26nMAssay Description:Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus systemMore data for this Ligand-Target Pair
TargetGenome polyprotein(Human rhinovirus 14)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
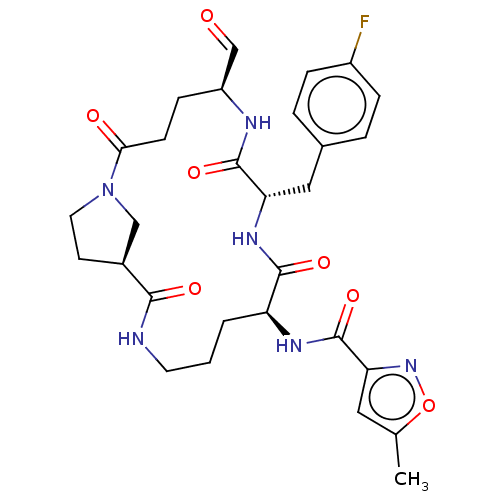
Affinity DataIC50: 2nMAssay Description:Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra...More data for this Ligand-Target Pair
TargetGenome polyprotein(Human rhinovirus 14)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
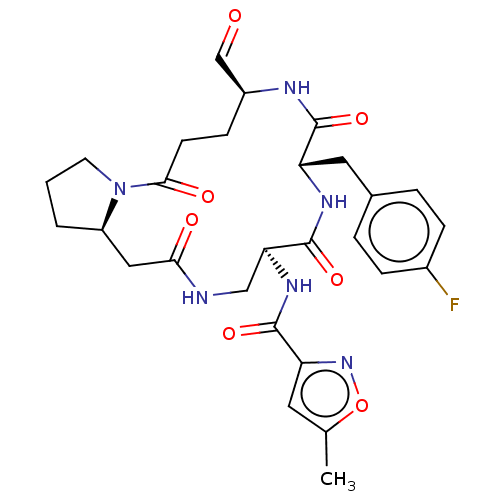
Affinity DataIC50: 2nMAssay Description:Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra...More data for this Ligand-Target Pair
TargetGenome polyprotein(Human rhinovirus 14)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of Human rhinovirus serotype 14 3C protease preincubated for 1 hr followed by Cys(PT14M)-Ala-Ile-Phe-Gln'Gly-Pro-Asp-Phe(4-NH2)-OH substra...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus systemMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus systemMore data for this Ligand-Target Pair

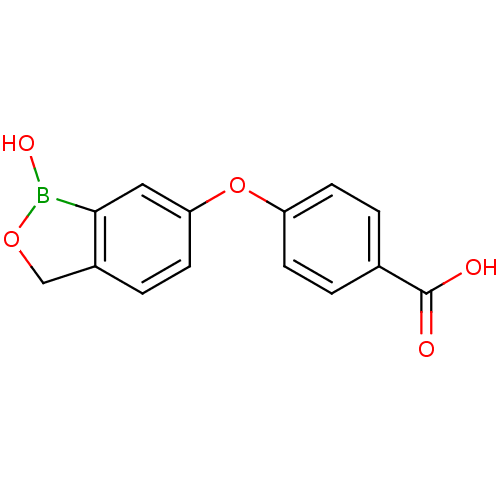
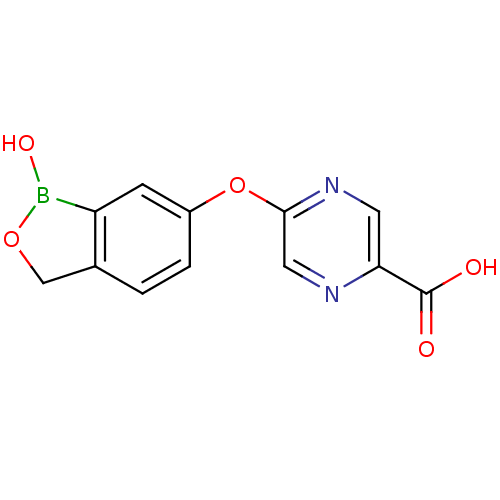
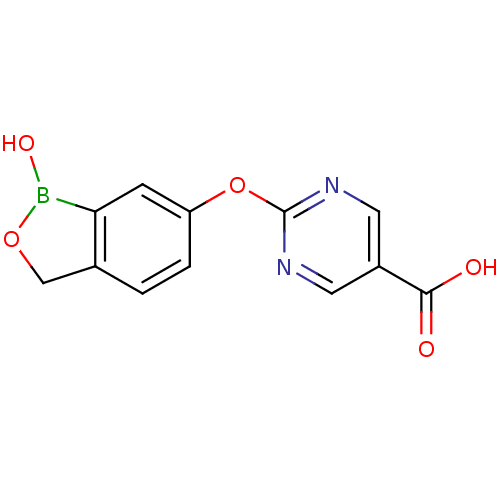
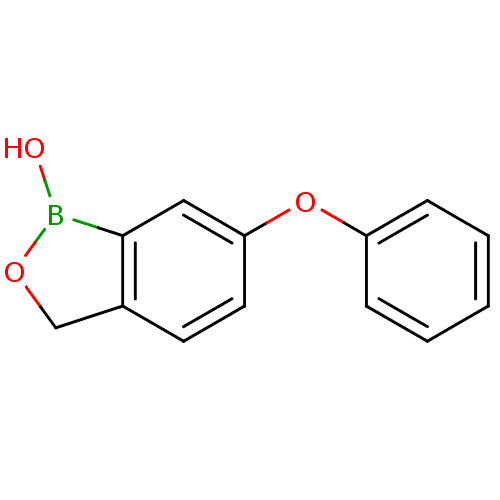

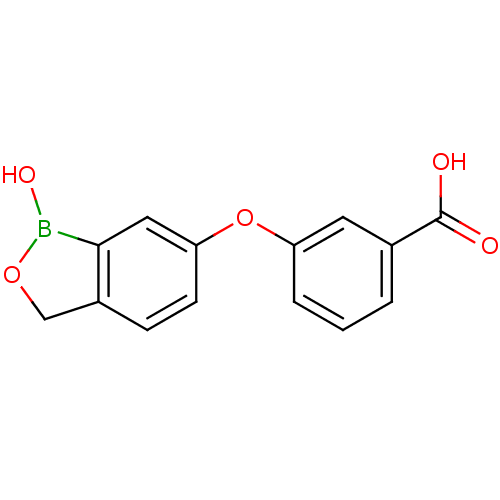
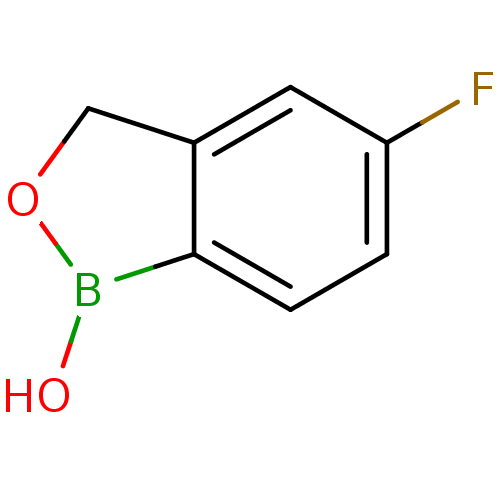
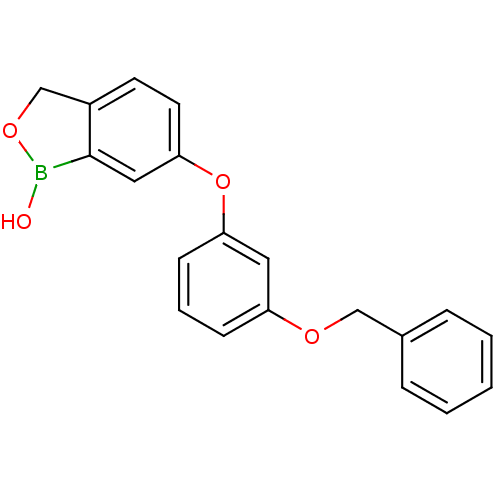

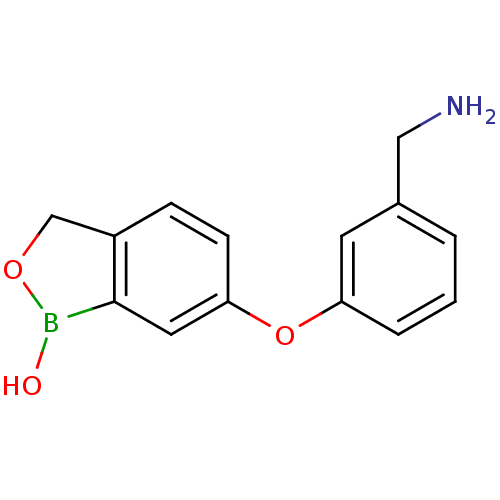

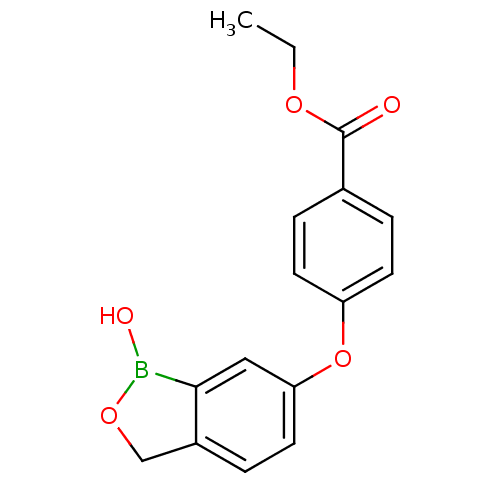


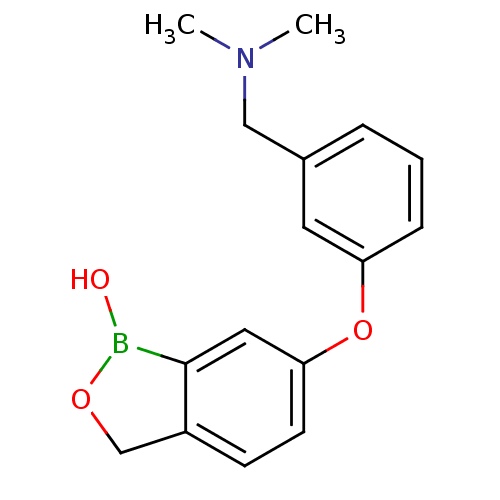




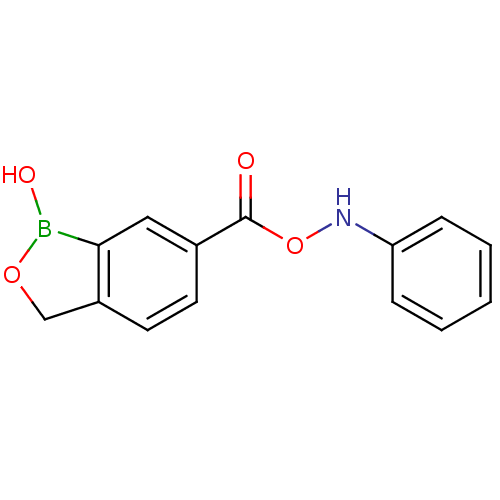
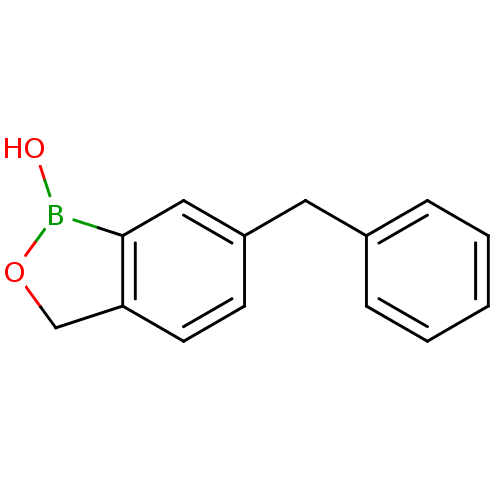
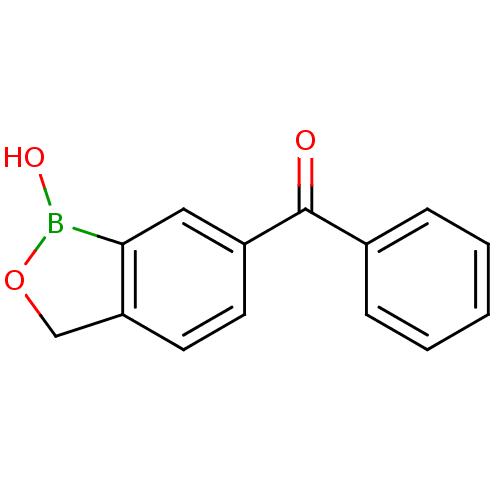


 3D Structure (crystal)
3D Structure (crystal)