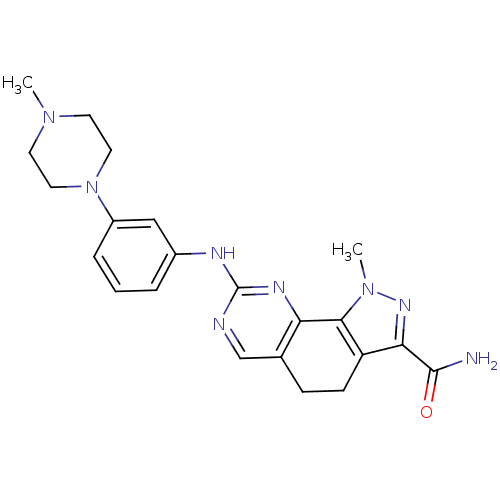
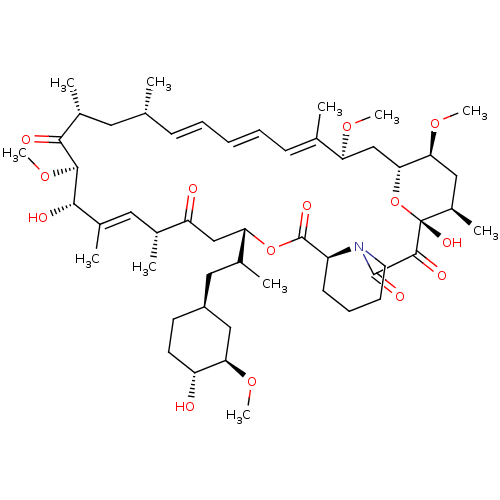
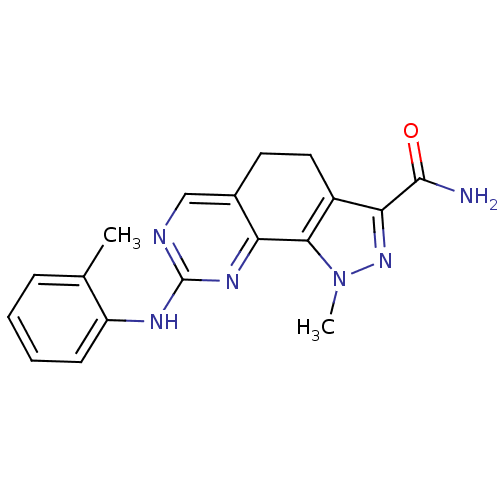
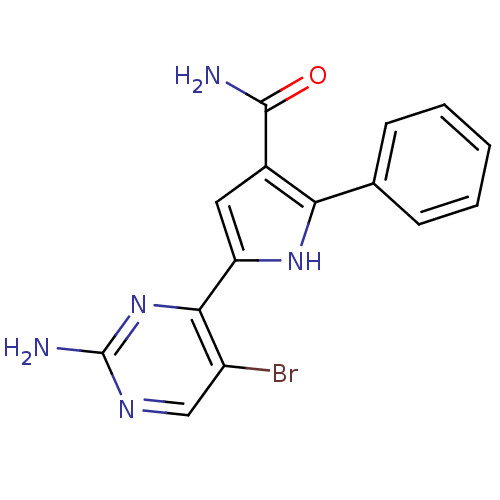
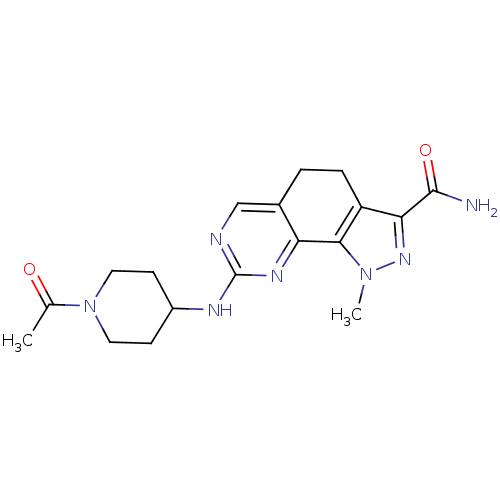
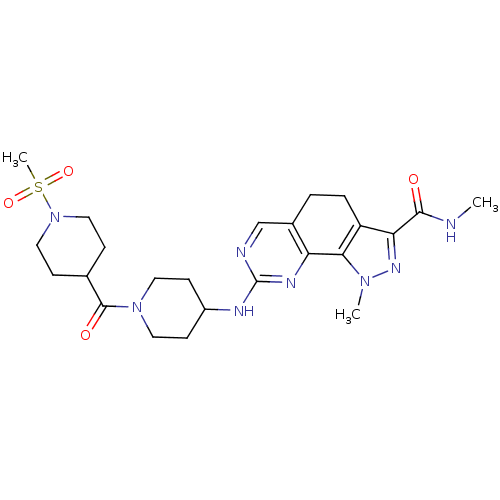
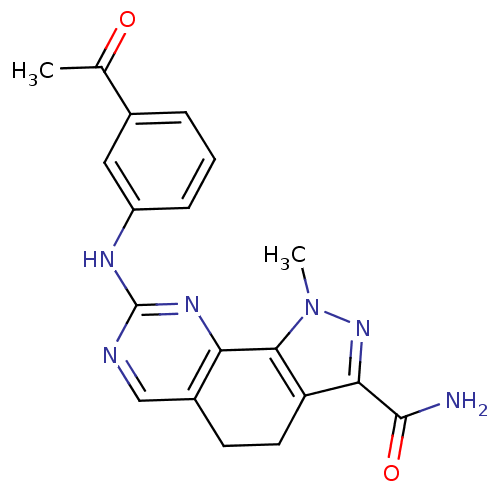
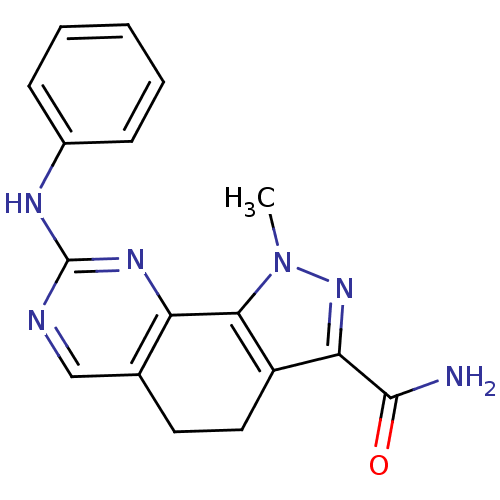
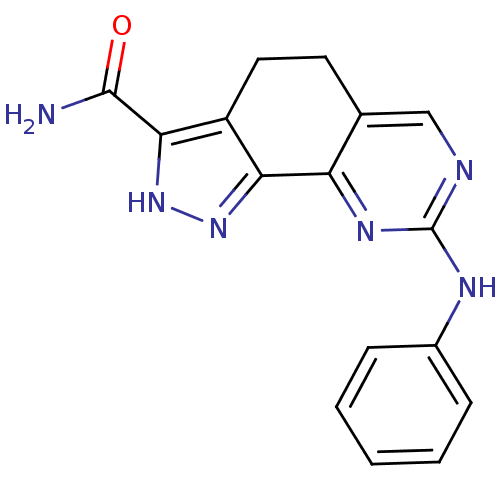
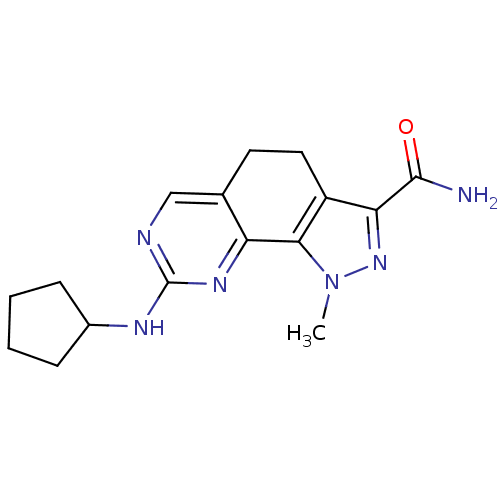
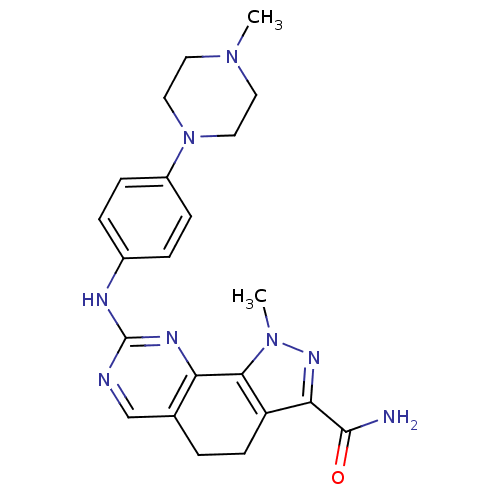
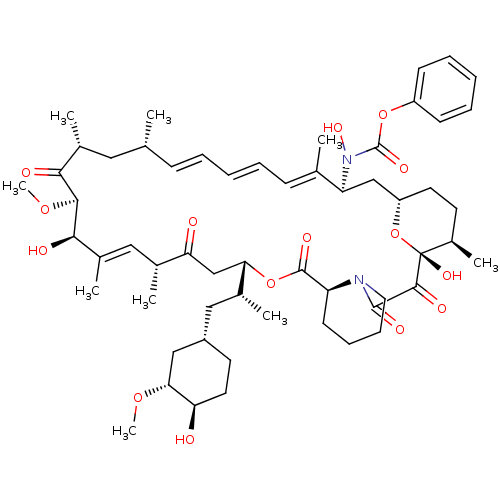
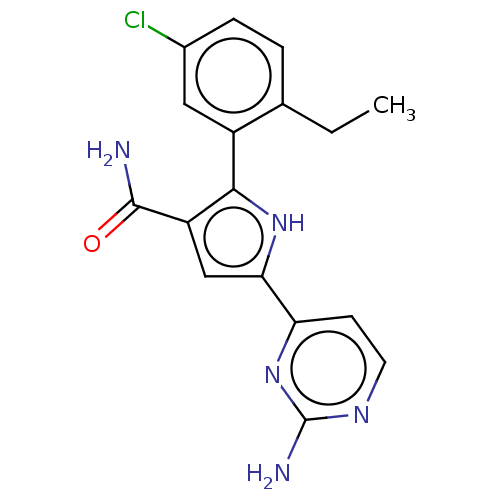
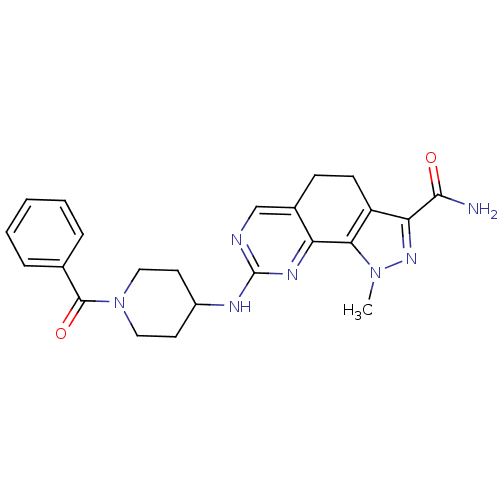
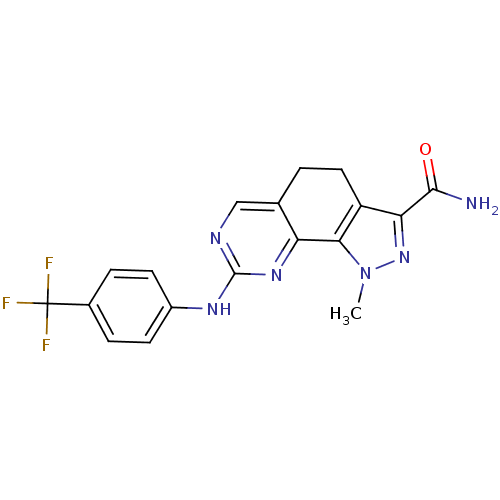
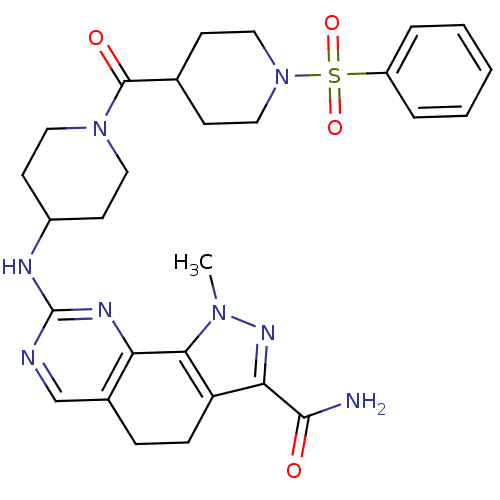
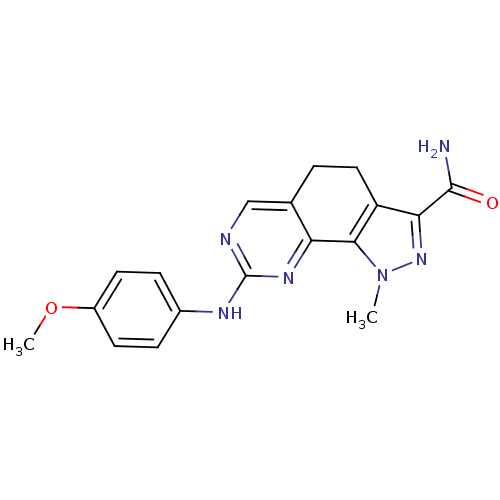
Affinity DataKi: 0.170nMAssay Description:Displacement of [3H]prazosin from human Alpha-1D adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:Displacement of [3H]prazosin from human Alpha-1D adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.324nMAssay Description:Displacement of [3H]prazosin from human Alpha-1B adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.417nMAssay Description:Displacement of [3H]prazosin from human Alpha-1B adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.490nMAssay Description:Displacement of [3H]prazosin from human Alpha-1D adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.537nMAssay Description:Displacement of [3H]prazosin from human alpha1A adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.813nMAssay Description:Displacement of [3H]prazosin from human Alpha-1D adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.813nMAssay Description:Displacement of [3H]prazosin from human Alpha-1B adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.933nMAssay Description:Displacement of [3H]prazosin from human Alpha-1B adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.41nMAssay Description:Displacement of [3H]prazosin from human Alpha-1B adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.58nMAssay Description:Displacement of [3H]prazosin from human Alpha-1D adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.86nMAssay Description:Displacement of [3H]prazosin from human alpha1A adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.82nMAssay Description:Displacement of [3H]prazosin from human alpha1A adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.39nMAssay Description:Displacement of [3H]prazosin from human alpha1A adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5.5nMAssay Description:Displacement of [3H]prazosin from human alpha1A adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 22.4nMAssay Description:Displacement of [3H]prazosin from human Alpha-1B adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 26nM ΔG°: -42.9kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair
Affinity DataKi: 26.3nMAssay Description:Displacement of [3H]prazosin from human Alpha-1D adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 33nM ΔG°: -42.3kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair
Affinity DataKi: 71nM ΔG°: -40.4kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair
Affinity DataKi: 81.3nMAssay Description:Displacement of [3H]prazosin from human alpha1A adrenoceptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 120nM ΔG°: -39.1kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nM ΔG°: -33.3kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nM ΔG°: -31.2kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of human recombinant DNA ligase 1 using nicked DNA substrate by kinetic assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.13E+4nM ΔG°: -28.0kJ/molepH: 4.5 T: 2°CAssay Description:Compounds were tested for their ability to inhibit BACE-1 hydrolysis of the internally quenched fluorescent substrate FS-2. Reactions were initiated ...More data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of CDK2/Cyclin AMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of CDK2/Cyclin AMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:The compound was tested for binding afifnity to FK506 binding protein 12 with an ascomycin conjugate of alkaline phosphatase in a competition binding...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant human JAK2 (808-1132 residues) using LPLDKDYYVVREPGQ as substrate by radiometric assay in presence of [33P]-gamma-ATPMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase PLK2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of Plk2 assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma countingMore data for this Ligand-Target Pair
TargetCell division cycle 7-related protein kinase(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of CDK2/Cyclin AMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of CDK2/Cyclin AMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma countingMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma countingMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma countingMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of CDK2/Cyclin AMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with specific enzymes and substrates, followed by quantitation of the phosphorylat...More data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma countingMore data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase FKBP1A(Homo sapiens (Human))
Abbott Laboratories
Curated by ChEMBL
Abbott Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:The compound was tested for binding afifnity to FK506 binding protein 12 using Rapamycin as control, with an ascomycin conjugate of alkaline phosphat...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant human JAK2 (808-1132 residues) using LPLDKDYYVVREPGQ as substrate by radiometric assay in presence of [33P]-gamma-ATPMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of CDK2/Cyclin AMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma countingMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma countingMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma countingMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of CDK2/Cyclin AMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Homo sapiens (Human))
Nerviano Medical Sciences
Curated by ChEMBL
Nerviano Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of CDK2/Cyclin A assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma countingMore data for this Ligand-Target Pair

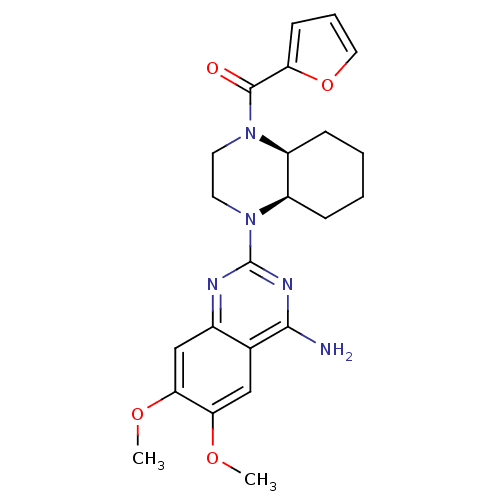
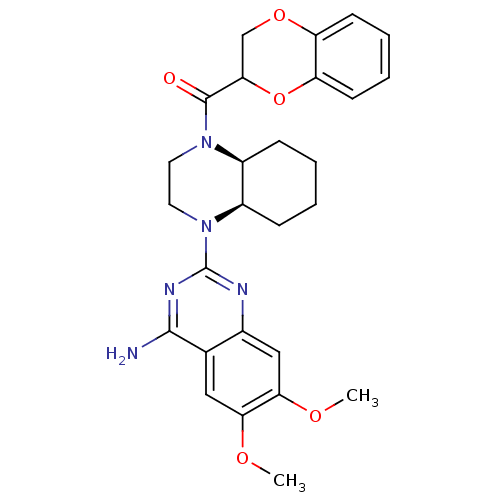
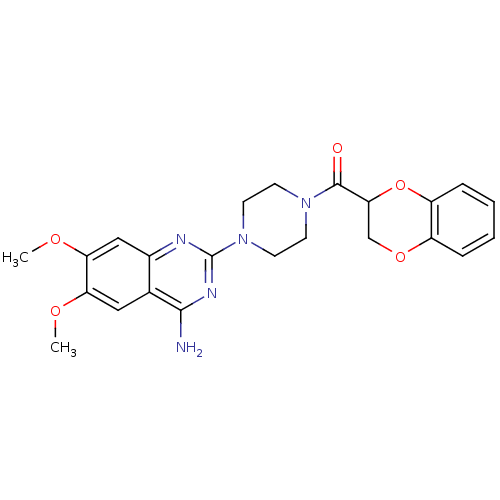
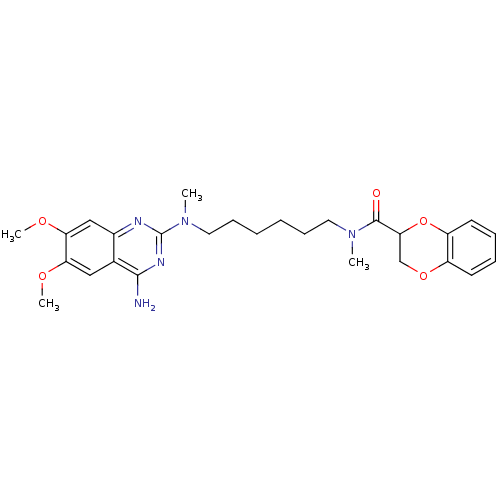
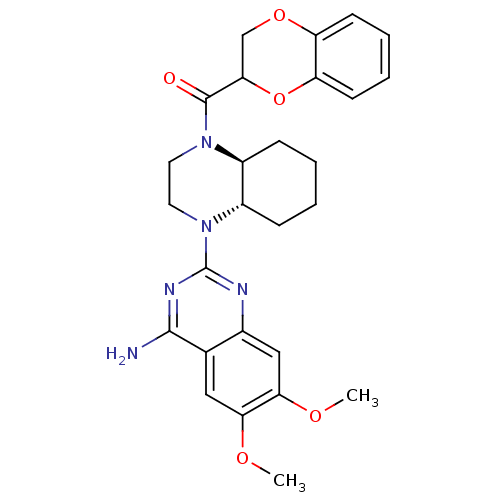
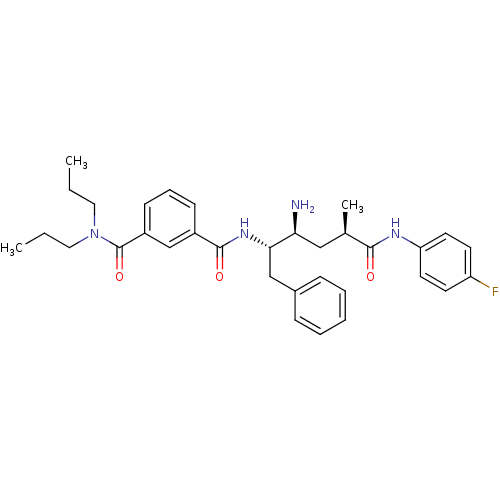
 3D Structure (crystal)
3D Structure (crystal)